
In a groundbreaking research, scientists from the Weizmann Institute of Science have developed a novel method to understanding molecular interactions, impressed by the intricate mechanics of cone snail toxins. This method transcends conventional methodologies, harnessing the ability of synthetic intelligence to unveil the complicated relationships between toxins and their organic targets. The findings, which shall be offered on the upcoming 69th Biophysical Society Annual Assembly in February 2025, may have far-reaching implications for each ecological analysis and the event of therapeutic medication.
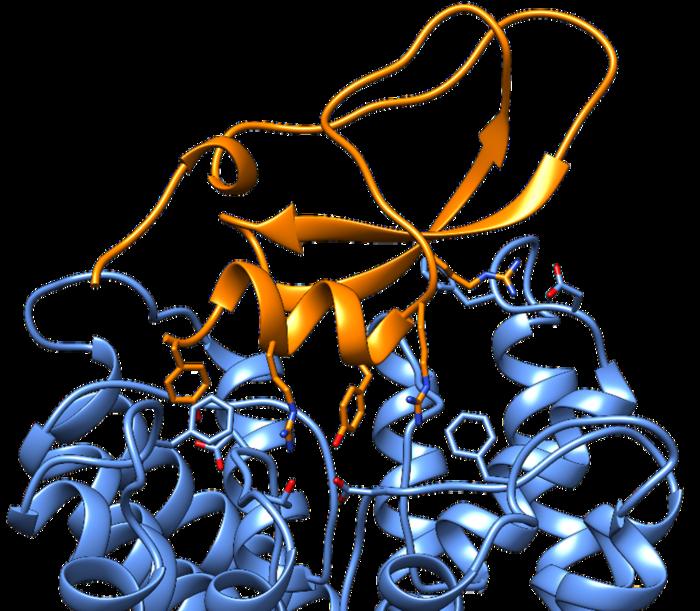
On the coronary heart of this analysis is the cone snail toxin referred to as Conkunitzin-S1 (Cs1). This toxin, primarily impacting potassium channels within the cells of fish and bugs, poses a novel problem for scientists searching for to grasp its exact mechanisms of motion. Whereas it’s well-documented that Cs1 successfully blocks potassium channels, rendering them unable to facilitate important mobile capabilities, the precise targets inside fish had remained elusive till now. Understanding these interactions is pivotal, not just for insights into ecological dynamics but in addition for drug growth purposes.
The analysis staff, led by Izhar Karbat and Eitan Reuveny, confronted vital hurdles when making an attempt to pinpoint the targets of Cs1 utilizing standard instruments three years in the past. Regardless of their greatest efforts, they might not attain the readability required to determine a complete understanding of the toxin’s interactions. Nevertheless, the arrival of superior AI applied sciences has revolutionized their method, enabling them to discover molecular interactions with unprecedented precision.
Using the AI program AlphaFold, the scientists first predicted the binding interactions between Cs1 and an array of fish potassium channels. This step was essential, because it supplied a foundational understanding of which channels may be impacted by the toxin. By leveraging AlphaFold’s capabilities, Reuveny and Karbat laid the groundwork for a deeper evaluation of those molecular interactions, enabling them to hypothesize how Cs1 engages with particular proteins.
Along with utilizing AlphaFold, the researchers developed ET3, an progressive AI mannequin designed to investigate the dynamics of water molecules surrounding potassium channels. This mannequin focuses on the selectivity filter—the a part of the channel liable for regulating ionic flux—understanding that disruptions on this area can result in channel inactivation. ET3, skilled on a large assortment of potassium channels, excels at figuring out anomalies in water motion, thereby illuminating potential binding websites for Cs1.
By this twin method, the analysis staff was capable of sift by means of an enormous panorama of potassium channels beforehand unexplored by standard strategies. Their findings revealed the precise fish potassium channels that Cs1 targets, shedding gentle on the intricate dynamics of the toxin’s interplay. The analysis illustrates that Cs1 capabilities akin to a lock that seizes management of those ion gates, stopping potassium from traversing the channel.
Moreover, Karbat expressed pleasure over the broader purposes of this analysis extending past the fast ecological implications. The pipeline established by means of their work opens new avenues for drug discovery, providing a option to precisely decide the targets of newly developed medication primarily based on their structural traits. Such precision is especially very important because it helps mitigate unintended uncomfortable side effects, similar to a drug meant for mind channels inadvertently affecting cardiac channels.
This analysis additionally highlights the significance of understanding off-target interactions, particularly in therapeutic contexts. As an example, if a drug developed to stimulate a potassium channel in neuronal tissues additionally prompts comparable channels in cardiac tissues, the implications may very well be extreme. Thus, the flexibility to precisely determine and differentiate targets presents a vital stride in guaranteeing drug security.
Furthermore, the implications of the findings prolong into ecological research. By using the newfound understanding of molecular interactions, researchers can delve deeper into ecological techniques and the roles performed by varied toxins inside them. This might result in insights about how these interactions have an effect on populations, ecosystems, and finally, biodiversity and conservation efforts.
In conclusion, the Weizmann Institute staff’s progressive mix of synthetic intelligence and conventional strategies has marked a big milestone within the subject of molecular biology. The analysis not solely enriches our understanding of cone snail toxins but in addition showcases the potential for AI to rework drug growth methods and ecological analysis. As scientists proceed to unravel the complexities of molecular interactions, this work stands as a testomony to the ability of interdisciplinary methods in advancing scientific inquiry.
Topic of Analysis: Interactions of Cone Snail Toxin with Potassium Channels
Article Title: Weizmann Institute Scientists Unravel Potassium Channel Interactions of Cone Snail Toxin Utilizing AI
Information Publication Date: TBD
Net References: TBD
References: TBD
Picture Credit: Courtesy of Eitan Reuveny and Izhar Karbat
Key phrases: Biophysics, Toxins, Molecular Biology, Synthetic Intelligence, Drug Growth
Tags: synthetic intelligence in biologybiophysical society annual meetingcone snail venom researchConkunitzin-S1 toxicityecological implications of toxinsinnovative scientific methodologiesmolecular biology advancementsmolecular interplay studiespotassium channel blockerstherapeutic drug developmentunderstanding toxin mechanismsWeizmann Institute of Science analysis




