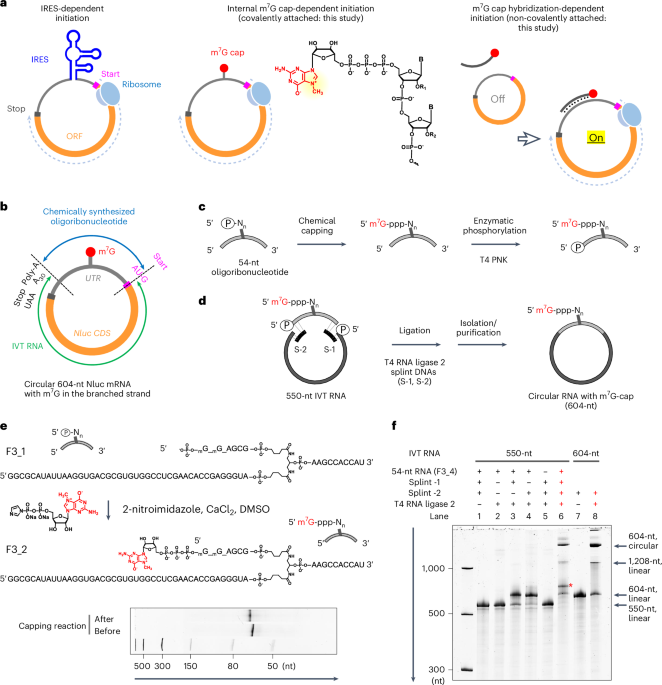
Polack, F. P. et al. Security and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and security of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Rohner, E., Yang, R., Foo, Okay. S., Goedel, A. & Chien, Okay. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Sparmann, A. & Vogel, J. RNA-based medication: from molecular mechanisms to remedy. EMBO J. 42, e114760 (2023).
Liu, C. et al. mRNA-based most cancers therapeutics. Nat. Rev. Most cancers 23, 526–543 (2023).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and may prolong translation length in vivo. Mol. Cell 74, 508–520 (2019).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering round RNA for potent and steady translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Meganck, R. M. et al. Tissue-dependent expression and translation of round RNAs with recombinant AAV vectors in vivo. Mol. Ther. Nucleic Acids 13, 89–98 (2018).
Meganck, R. M. et al. Engineering extremely environment friendly backsplicing and translation of artificial circRNAs. Mol. Ther. Nucleic Acids 23, 821–834 (2021).
Qu, L. et al. Round RNA vaccines towards SARS-CoV-2 and rising variants. Cell 185, 1728–1744 (2022).
Chen, R. et al. Engineering round RNA for enhanced protein manufacturing. Nat. Biotechnol. 41, 262–272 (2023).
Unti, M. J. & Jaffrey, S. R. Extremely environment friendly mobile expression of round mRNA allows extended protein expression. Cell Chem. Biol. 31, 163–176 (2024).
Amaya, L. et al. Round RNA vaccine induces potent T cell responses. Proc. Natl Acad. Sci. USA 120, e2302191120 (2023).
Pelletier, J., Schmeing, T. M. & Sonenberg, N. The multifaceted eukaryotic cap construction. Wiley Interdiscip. Rev. RNA 12, e1636 (2021).
Pelletier, J. & Sonenberg, N. The organizing ideas of eukaryotic ribosome recruitment. Annu. Rev. Biochem. 88, 307–335 (2019).
Wang, Y. & Wang, Z. F. Environment friendly backsplicing produces translatable round mRNAs. RNA 21, 172–179 (2015).
Yang, Y. et al. Intensive translation of round RNAs pushed by N6-methyladenosine. Cell Res. 27, 626–641 (2017).
He, P. C. & He, C. m6A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 40, e105977 (2021).
Hellen, C. U. T. IRES-induced conformational adjustments within the ribosome and the mechanism of translation initiation by inside ribosomal entry. Biochim. Biophys. Acta 1789, 558–570 (2009).
Corridor, M. P. et al. Engineered luciferase reporter from a deep sea shrimp using a novel imidazopyrazinone substrate. ACS Chem. Biol. 7, 1848–1857 (2012).
Abe, N. et al. Full chemical synthesis of minimal messenger RNA by environment friendly chemical capping response. ACS Chem. Biol. 17, 1308–1314 (2022).
Ellipilli, S., Phillips, J. D. & Heemstra, J. M. Synthesis of comb-shaped DNA utilizing a non-nucleosidic branching phosphoramidite. Org. Biomol. Chem. 16, 4659–4664 (2018).
Abe, N. et al. Rolling circle translation of round RNA in residing human cells. Sci. Rep. 5, 16435 (2015).
Karikó, Okay., Buckstein, M., Ni, H. P. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the affect of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Karikó, Okay. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with elevated translational capability and organic stability. Mol. Ther. 16, 1833–1840 (2008).
Kao, C., Zheng, M. & Rüdisser, S. A easy and environment friendly technique to scale back nontemplated nucleotide addition on the 3′ terminus of RNAs transcribed by T7 RNA polymerase. RNA 5, 1268–1272 (1999).
Santoro, S. W. & Joyce, G. F. A normal function RNA-cleaving DNA enzyme. Proc. Natl Acad. Sci. USA 94, 4262–4266 (1997).
Gamper, H. et al. Synthesis of lengthy RNA with a site-specific modification by enzymatic splint ligation. Preprint at bioRxiv https://doi.org/10.1101/2022.09.17.508400 (2022).
Palmenberg, A. C., Rathe, J. A. & Liggett, S. B. Evaluation of the entire genome sequences of human rhinovirus. J. Allergy Clin. Immunol. 125, 1190–1199 (2010).
Bordeleau, M. E. et al. Purposeful characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol. 2, 213–220 (2006).
Steinberger, J. et al. Identification and characterization of hippuristanol-resistant mutants reveals eIF4A1 dependencies inside mRNA 5′ chief areas. Nucleic Acids Res. 48, 9521–9537 (2020).
Abdullah, S. W., Wu, J. E., Wang, X. F., Guo, H. C. & Solar, S. Q. Advances and breakthroughs in IRES-directed translation and replication of picornaviruses. mBio 14, e0035823 (2023).
Bernstein, P., Peltz, S. W. & Ross, J. The poly(A)-poly(A)-binding protein advanced is a serious determinant of mRNA stability in vitro. Mol. Cell. Biol. 9, 659–670 (1989).
Deo, R. C., Bonanno, J. B., Sonenberg, N. & Burley, S. Okay. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell 98, 835–845 (1999).
Henderson, J. M. et al. Cap 1 messenger RNA synthesis with co-transcriptional CleanCap(®) analog by in vitro transcription. Curr. Protoc. 1, e39 (2021).
Zeng, C., Zhang, C., Walker, P. G. & Dong, Y. Formulation and supply applied sciences for mRNA vaccines. Curr. Prime. Microbiol. Immunol. 440, 71–110 (2022).
Husseini, R. A., Abe, N., Hara, T., Abe, H. & Kogure, Okay. Use of iontophoresis expertise for transdermal supply of a minimal mRNA vaccine as a possible melanoma therapeutic. Biol. Pharm. Bull. 46, 301–308 (2023).
Imani, S., Tagit, O. & Pichon, C. Neoantigen vaccine nanoformulations primarily based on Chemically synthesized minimal mRNA (CmRNA): small molecules, large affect. NPJ Vaccines 9, 14 (2024).
Puttaraju, M. et al. Systematic screening identifies therapeutic antisense oligonucleotides for Hutchinson–Gilford progeria syndrome. Nat. Med. 27, 526–535 (2021).
Lu, Z. J. & Mathews, D. H. Elementary variations within the equilibrium issues for siRNA and antisense oligodeoxynucleotide design. Nucleic Acids Res. 36, 3738–3745 (2008).
Egli, M. & Manoharan, M. Chemistry, construction and performance of authorized oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023).
Lesiak, Okay., Khamnei, S. & Torrence, P. F. 2′,5′-Oligoadenylate:antisense chimeras. Synthesis and properties. Bioconjugate Chem. 4, 467–472 (1993).
Passmore, L. A. & Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 23, 93–106 (2022).
Picard-Jean, F. et al. 2′-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS ONE 13, e0193804 (2018).
Despic, V. & Jaffrey, S. R. mRNA ageing shapes the Cap2 methylome in mammalian mRNA. Nature 614, 358–366 (2023).
Koshkin, A. A. et al. LNA (locked nucleic acids): synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 54, 3607–3630 (1998).
Obika, S. et al. Stability and structural options of the duplexes containing nucleoside analogues with a hard and fast N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett. 39, 5401–5404 (1998).
Costello, A., Lao, N. T., Barron, N. & Clynes, M. Steady translation of circularized mRNA improves recombinant protein titer. Metab. Eng. 52, 284–292 (2019).
Mattick, J. S. et al. Lengthy non-coding RNAs: definitions, features, challenges and proposals. Nat. Rev. Mol. Cell Biol. 24, 430–447 (2023).
Yu, X., Zheng, H. Y., Chan, M. T. V. & Wu, W. Okay. Okay. HULC: an oncogenic lengthy non-coding RNA in human most cancers. J. Cell. Mol. Med. 21, 410–417 (2017).
Zhao, Y. et al. Position of lengthy non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric most cancers: a scientific and in vitro investigation. Oncol. Rep. 31, 358–364 (2014).
Joseph, R., Srivastava, O. P. & Pfister, R. R. Downregulation of β-actin and its regulatory gene HuR have an effect on cell migration of human corneal fibroblasts. Mol. Vis. 20, 593–605 (2014).
Morais, P., Adachi, H. & Yu, Y. T. The crucial contribution of pseudouridine to mRNA COVID-19 vaccines. Entrance. Cell Dev. Biol. 9, 789427 (2021).
Bathula, N. V. et al. Supply car and route of administration influences self-amplifying RNA biodistribution, expression kinetics, and reactogenicity. J. Management. Launch 374, 28–38 (2024).
Anderluzzi, G. et al. The position of nanoparticle format and route of administration on self-amplifying mRNA vaccine efficiency. J. Management. Launch 342, 388–399 (2022).
Münter, R., Christensen, E., Andresen, T. L. & Larsen, J. B. Learning how administration route and dose regulates antibody technology towards LNPs for mRNA supply with single-particle decision. Mol. Ther. Strategies Clin. Dev. 29, 450–459 (2023).
Darrah, P. A. et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102 (2020).
Leppek, Okay., Das, R. & Barna, M. Purposeful 5′ UTR mRNA constructions in eukaryotic translation regulation and how you can discover them. Nat. Rev. Mol. Cell Biol. 19, 158–174 (2018).
Paek, Okay. Y., Park, S. M., Hong, Okay. Y. & Jang, S. Okay. Cap-dependent translation with out base-by-base scanning of an messenger ribonucleic acid. Nucleic Acids Res. 40, 7541–7551 (2012).
Carrieri, C. et al. Lengthy non-coding antisense RNA controls Uchl1 translation by an embedded SINEB2 repeat. Nature 491, 454–457 (2012).
Pierattini, B. et al. SINEUP non-coding RNA exercise is dependent upon particular N6-methyladenosine nucleotides. Mol. Ther. Nucleic Acids 32, 402–414 (2023).
Sharma, H. et al. Decryption of sequence, construction, and practical options of SINE repeat parts in SINEUP non-coding RNA-mediated post-transcriptional gene regulation. Nat. Commun. 15, 1400 (2024).
Cao, Y. et al. RNA-based translation activators for focused gene upregulation. Nat. Commun. 14, 6827 (2023).
Cao, X. A., Zhang, Y. Y., Ding, Y. L. & Wan, Y. Identification of RNA constructions and their roles in RNA features. Nat. Rev. Mol. Cell Biol. 25, 784–801 (2024).
Solar, L. et al. RNA construction maps throughout mammalian mobile compartments. Nat. Struct. Biol. 26, 322–330 (2019).
Liu, C. X. & Chen, L. L. Round RNAs: characterization, mobile roles, and purposes. Cell 185, 2016–2034 (2022).
Diallo, L. H. et al. How are circRNAs translated by non-canonical initiation mechanisms? Biochimie 164, 45–52 (2019).
Legnini, I. et al. Circ-ZNF609 is a round RNA that may be translated and features in myogenesis. Mol. Cell 66, 22–37 (2017).
Pitsch, S., Weiss, P. A., Jenny, L., Stutz, A. & Wu, X. L. Dependable chemical synthesis of oligoribonucleotides (RNA) with 2′-O-[(triisopropylsilyl)oxy]methyl(2′-O-tom)-protected phosphoramidites. Helv. Chim. Acta 84, 3773–3795 (2001).
Ototake, M. et al. Improvement of hydrophobic tag purifying monophosphorylated RNA for chemical synthesis of capped mRNA and enzymatic synthesis of round mRNA. Nucleic Acids Res. 52, 12141–12157 (2024).
Inagaki, M. et al. Cap analogs with a hydrophobic photocleavable tag allow facile purification of totally capped mRNA with numerous cap constructions. Nat. Commun. 14, 2657 (2023).