Commercially out there supplies
Until in any other case acknowledged, all reagents and reagent-grade solvents had been bought from Sigma-Aldrich (Milwaukee, WI) and used as obtained. 3-(Stearoyloxy)-2,2-bis[(stearoyloxy)methyl]propyl stearate (PE-S4) was both synthesized in high-purity (see beneath) or bought from Sigma-Aldrich (Milwaukee, WI) as a mix of PE-S4 and incomplete-substitution merchandise (PE-S3 and PE-S2). The phospholipid, 1,2-dipalmitoryl-sn-glycero-3-phosphocholine (DPPC) was bought from Avanti Polar Lipids (Alabaster, AL). Human apolipoprotein A-I (apoA-I) protein, human serum-derived HDL2 and HDL3 subfractions and TGF-β1 protein had been bought from MyBioSource (San Diego, CA). Extremely-pure deionized (DI) water (18.2 MΩ-cm resistivity) was obtained from a Millipore Synergy UV system whereas nuclease-free water was obtained from Cytiva Life Sciences. Dulbecco’s Phosphate Buffered Saline (DPBS, 1X) was bought from Corning.
Instrumentation
Absorption measurements for BCA, NF-κB reporter, phospholipid quantification, and RNA oligonucleotide quantification assays had been recorded with a Synergy 2 microplate reader (BioTek).
Synthesis of PE-S4 and PE-O4 core scaffolds
For this work, we employed two oc molecules to synthesize optically clear oc-HDL NPs. We utilized one core with unsaturated fatty acyl chains emanating from a central core, pentaerythritol tetraoleate (PE-O4), and one with totally saturated fatty acyl chains, pentaerythritol tetrastearate (PE-S4), to behave as scaffolds for the meeting of lipids and apolipoprotein A-I to kind PE-O4 HDL NP and PE-S4 HDL NP, respectively. Each of the oc molecules will be made in large-scale and economical style.
Synthesis of pure tetraoleyl pentaerythritol (PE-O4) utilizing in-situ-generated oleyl chloride
In a 100 mL flame-dried round-bottom flask geared up with a magnetic stir bar, oleic acid (3.2 mL, 10 mmol, 10 equiv) and anhydrous DCM (10 mL, 0.1 M) had been mixed underneath a nitrogen ambiance utilized by a balloon on prime of the round-bottom flask. The ensuing combination was positioned in an ice bathtub for 10 min. One drop of DMF was added because the catalyst, adopted by the addition of oxalyl chloride (1.8 mL, 20 mmol, 20 equiv). A fine-gauged needle was positioned on prime of the round-bottom flask to permit for the venting of generated HCl fuel in the course of the preliminary a part of the response. After fuel evolution ceased, the ice bathtub was eliminated, and the response combination was allowed to heat as much as room temperature and stirred at ambient temperature for 3 h, throughout which era the nitrogen ambiance was sometimes replenished by re-inflating the balloon. After the response, the risky parts had been then eliminated on a Schlenk line; anhydrous DCM was then added to the ensuing crude product to redissolve it for the subsequent step.
The round-bottom flask containing the DCM answer of the in-situ-generated oleyl chloride was capped with a rubber septum and positioned in an ice bathtub to chill right down to 0 °C. Below a nitrogen ambiance utilized by a balloon on prime of the flask, pentaerythritol (0.14 g, 1 mmol, 1 equiv) and pyridine (0.8 mL, 10 mmol, 10 equiv) had been added neat. The ensuing response combination was stirred at room temperature for twenty-four h. The response combination was poured right into a separation funnel and washed with NaOH (3 ×10 mL of a 0.1 M aqueous answer) and HCl (10 mL of a 0.1 M aqueous answer). The remaining natural layer was collected, dried over Na2SO4, and evaporated to dryness underneath lowered stress. The crude product was then subjected to column chromatography (30 mm × 250 mm silica gel, hexanes/DCM eluents at 10:1, 3:1, 2:1, and 1:1 ratio). This course of afforded the specified compound as a yellow oil after isolation (0.4 g, 0.34 mmol, 34% yield). 1H NMR (500 MHz, CDCl3): δ 0.89 (d, J = 6.7 Hz, 12H), 1.28 (dt, J = 14.9, 4.8 Hz, 91H), 1.51-1.66 (m, 13H), 2.01 (q, J = 6.6 Hz, 16H), 2.30 (t, J = 7.6 Hz, 8H), 4.11 (s, 8H), 5.27-5.44 (m, 8H). 13C NMR (126 MHz, CDCl3): δ 14.3, 22.8, 25.0, 27.3, 27.4, (a number of intently spaced alkyl peaks: 29.27, 29.28, 29.33, 29.47, 29.48, 29.68, 29.86, 29.92), 32.1, 34.3, 42.0, 62.3, 76.9, 77.2, 77.4, 129.9, 130.2, 173.4.
Synthesis of pure tetraoleyl pentaerythritol (PE-O4) utilizing commercially out there oleyl chloride
In a 100 mL flame-dried round-bottom flask geared up with a magnetic stir bar and hooked up to a Schlenk line, oleoyl chloride (3.61 g, 12 mmol, 6 equiv) and pentaerythritol (0.272 g, 2 mmol, 1 equiv) had been mixed underneath a nitrogen ambiance. The ensuing response combination was then stirred at 120 °C underneath dynamic vacuum for 1 h after which static vacuum for 3 extra h. The crude response combination was then cooled to room temperature, diluted with DCM (15 mL), mixed with aqueous NaOH (30 mL of a 1 M answer), and stirred for 15 min earlier than being poured right into a separation funnel. The aqueous layer, which incorporates some solids, was separated from the natural, filtered, and extracted with DCM (2 ×15 mL). The DCM extract was then washed with saturated brine (30 mL), mixed with the earlier natural layer, dried over Na2SO4, and evaporated to dryness. The ensuing product was additional purified utilizing column chromatography (30 mm × 250 mm, hexane/DCM eluents at 3:1, 2:1, and 1:1 ratio). This course of afforded the specified pure compound as a yellow oil after isolation (1.4 g, 1.19 mmol, 61% yield). 1H NMR (500 MHz, CDCl3): δ 0.83–0.92 (m, 13H), 1.19–1.39 (m, 80H), 1.60 (t, J = 7.3 Hz, 8H), 2.01 (q, J = 6.6 Hz, 15H), 2.30 (t, J = 7.6 Hz, 8H), 4.11 (s, 8H), 5.34 (qd, J = 3.7, 1.6 Hz, 8H). 13 C NMR (126 MHz, CDCl3): δ 14.3, 22.8, 25.0, 27.4, (a number of intently spaced alkyl peaks: 29.28, 29.47, 29.68, 29.89), 32.1, 34.2, 42.0, 62.3, 129.86, 130.15, 173.36.
Synthesis of pure tetrastearyl pentaerythritol (PE-S4) core utilizing commercially out there stearoyl chloride
In a 100 mL flame-dried round-bottom flask geared up with a magnetic stir bar and hooked up to a Schlenk line, stearoyl chloride (3.64 g, 12 mmol, 6 equiv) and pentaerythritol (0.272 g, 2 mmol, 1 equiv) had been mixed underneath a nitrogen ambiance. The ensuing response combination was stirred at 120 °C underneath dynamic vacuum for 1 h after which static vacuum for 3 h. The crude response combination was then cooled to room temperature, diluted with DCM (15 mL), mixed with aqueous NaOH (30 mL of a 1 M), and stirred for 15 min earlier than being poured right into a separation funnel. The aqueous layer, which incorporates some solids, was separated from the natural, filtered, and extracted with DCM (2 × 15 mL). The DCM extract was then washed with saturated brine (30 mL), mixed with the earlier natural layer, dried over Na2SO4, and evaporated underneath lowered stress till a big quantity of the pure product started to precipitate out of answer. The precipitated compound was then remoted through filtration, rapidly washed over the filter with chilly DCM, collected, and dried underneath vacuum. This course of afforded the specified pure compound as a white waxy stable (1.5 g, 1.22 mmol, 64% yield). 1H NMR (500 MHz, CDCl3): δ 0.88 (t, J = 6.9 Hz, 12H), 1.25 (s, 109H), 1.59 (p, J = 7.3 Hz, 12H), 2.30 (t, J = 7.6 Hz, 8H), 4.11 (s, 8H). 13C NMR (126 MHz, CDCl3): δ 14.1, 22.7, 24.9, 29.2, 29.3, 29.5, 29.7, 29.7, 31.9, 34.1, 62.2, 173.3.
Meeting of oc-HDL NPs
Meeting of PE-S4 HDL NP and/or PE-O4 HDL NP was performed by first getting ready shares of DPPC (1 mM answer in dichloromethane (DCM)) and the natural core (both PE-S4 or PE-O4 as 0.1% w/v options in DCM). In what we name the “one pot methodology,” all of the parts are dissolved in solvent and mixed right into a single vial. From the DPPC inventory, a 200 nmol amount is transferred to a clear 2 mL glass vial. To this was then added 1 nmol amount of the natural core (both PE-S4 or PE-O4). Then, an aliquot containing 2 nmol of apoA-I dissolved in 110 μL of PBS was added to the glass vial. The combination within the glass vial was vortexed for 10 s and the glass vial was then positioned on ice for 15 min to chill down. The cooled-down combination was then sonicated in a Bransonic 1800 (Branson) lab sonicator for 1 min, adopted by 30 sec of relaxation; and this sonication-rest cycle was repeated 4 extra instances. After the ultimate spherical of sonication, the glass vial, now containing a milky white emulsion, was positioned on ice once more till prepared for the subsequent step (usually
The next day, the contents of the glass vials had been transferred to 1.5 mL Eppendorf tubes and centrifuged at 10,000 g for 10 min to take away any giant lipid assemblies which will have fashioned. The answer/ supernatants had been then transferred to pre-wetted Amicon Extremely 0.5 mL 50 kDa MWCO regenerated cellulose spin columns (Millipore) and centrifuged at 10,000 g for 10 min at 4 °C, after which the flow-through was discarded and the retentate (often 20-50 μL) was additional diluted with PBS (0.5 mL), homogenized, and centrifuge once more. This centrifugation-and-washing cycle was repeated no less than 3 instances to take away any un-assembled apoA-I, lipid, or core materials. After the ultimate wash, the retentate was dropped at a last quantity of 100 μL with PBS and filtered utilizing a regenerated cellulose syringe filter (4 mm, 0.2 μm pore dimension, Thermo Scientific). Following filtration, the oc HDL NPs shares had been both used instantly or saved at 4 °C.
Dynamic mild scattering (DLS)
DLS and zeta potential measurements had been performed on a Zetasizer Nano ZS geared up with a He-Ne laser (633 nm) utilizing non-invasive backscatter (173° scattering angle) for detection. For each DLS and zeta potential measurements, samples had been ready in DI water at 150 μM protein focus. For every pattern, three measurements had been recorded with 10 runs per measurement.
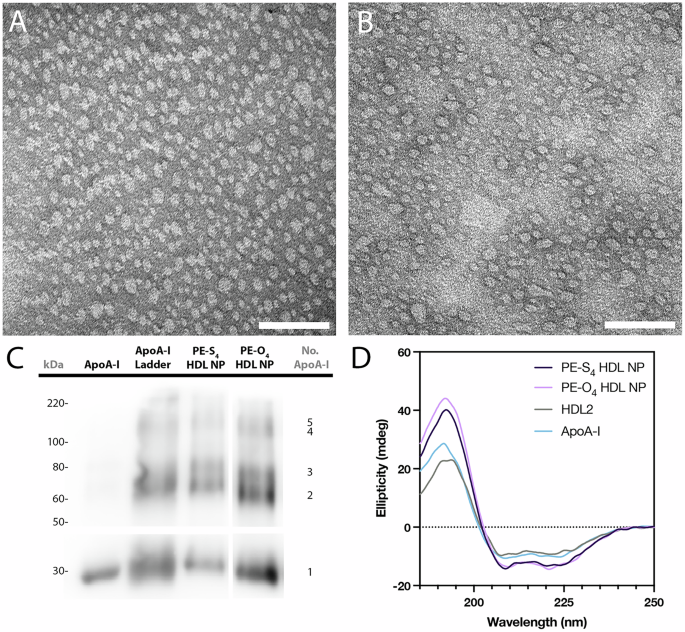
Adverse stain transmission digital microscopy
Copper grids had been first ready by treating in a plasma cleaner for 10 sec. Particles had been then diluted to an apoA-I focus of 250 μM in PBS and 20 μL of this answer was drop-cast onto plasma handled grids and left there for five min. Grids had been washed twice with PBS after which stained for 1 min in a 2% aqueous uranyl acetate answer. Lastly, the grids had been washed with water and air-dried earlier than imaging with a FEI Tecnai Spirit TEM working at 80 kV.
Round dichroism (CD) spectroscopy
Particles had been ready at an apoA-I focus of 15 μM in a ten mM monosodium phosphate buffer and analyzed in a 0.1 mm quartz cuvette (Jasco) on a J-1500 CD Spectrometer (Jasco). Spectra had been captured between 190 and 250 nm. Secondary construction evaluation was carried out utilizing the BeStSel software program, out there at https://bestsel.elte.hu/index.php.
In situ apoA-I crosslinking and western blot
In situ crosslinking of apoA-I used to be carried out to stabilize oligomerized apoA-I in ocHDL NP. The bis(sulfosuccinidimidyl)suberate (BS3, Sigma Aldrich) crosslinker was used for this objective in line with a beforehand reported protocol29. Briefly, ocHDL NP had been diluted to a last protein focus of fifty μg/mL in PBS. The BS3 reagent was then added to the particles (last focus = 2.5 mM). The combination was incubated at room temperature for 30 min earlier than the response was quenched by including 0.5 M Tris (last focus = 45 mM). The apoA-I oligomer ladder was generated by mixing PBS options of apoA-I (500 μg/mL) with BS3 (last focus = 0.25 mM) and incubating for 4 h at room temperature. The response was stopped by including 0.5 M Tris (last focus = 45 mM).
The apoA-I ladder and crosslinked particle samples had been run on a pre-cast 4-20% polyacrylamide gel (MiniProtean TGX, Bio-Rad). The protein was then transferred to a polyvinylidene fluoride (PVDF) membrane in tris-glycine buffer with 20% w/v methanol. Subsequent the membrane was rinsed with Tris-buffered saline (TBS) and blocked in 5% w/v nonfat dry milk diluted in TBS for 1 h at room temperature. A major anti-apoAI antibody (rabbit, Abcam) was added to the membrane at 1:1000 dilution in 5% w/v nonfat dry milk and incubated at 4 °C in a single day. The next day, the membrane was washed thrice for 10 min every in TBS-Tween (0.1%). An anti-rabbit HRP conjugated secondary antibody (goat, BioRad) was then added at a 1:2000 dilution in 5% w/v nonfat dry milk and incubated at room temperature for two h, after which the membrane was washed 3 thrice for 10 min every in TBS-Tween (0.1%). Lastly, the membrane was bathed in electrochemiluminescence substrate answer for 1 min and imaged on an C300 Gel Imager (Azure Biosystems).
Quantification of protein content material of artificial HDL NPs and native HDLs
The protein content material of particles and native HDL was decided utilizing a commercially out there bicinchoninic acid (BCA) assay (Thermo Fisher) utilizing the producer’s protocol. Briefly, a regular curve was generated in a 96-well plate by including bovine serum albumin (BSA) into aliquots of BCA reagent (last quantity = 80 μL) in duplicate, to realize concentrations starting from 0.125-2 mg/mL. Nanoparticle samples had been diluted into BCA reagent utilizing the identical methodology to generate a 1:16 dilution in triplicate. The plate was then incubated at 37 °C for 20 min after which the absorbance of every effectively was measured with a Synergy microplate reader at 562 nm. A linear regression was calculated for the usual curve utilizing the least squares methodology and protein focus of particle samples interpolated from the road of greatest match.
Phospholipid quantification
The phospholipid content material of nanoparticles and native HDL was quantified utilizing a commercially out there phospholipid quantification package (Sigma Aldrich) and per the producer’s protocols. Briefly, a calibration curve was generated in a 96-well plate by dilution of an included phospholipid customary to concentrations starting from 20-100 μM into DI water. Samples had been serially diluted in DI water, after which an enzyme/reagent combination was add to pattern and requirements. The plate was incubated for 30 min at 37 °C earlier than measuring the absorbance of every effectively at 570 nm with a Synergy microplate reader. Remaining phospholipid concentrations in samples had been interpolated from a linear customary curve generated utilizing the least squares methodology.
NF-κB exercise assays
For NF-κB exercise assays, THP1-Twin cells (Invivogen) had been cultured in a base media of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. After a number of passages, cells containing the NF-κB reporter advanced had been chosen (base media containing 10 μg/mL of Blasticidin and 100 μg/mL of Zeocin) previous to being exchanged into base media for experiments. The THP1-Twin cells had been plated right into a 96-well plate at 1 × 105 cells/effectively and stimulated with lipopolysaccharide (LPS) at (1 ng/mL) for twenty-four h. Experimental wells had been concurrently handled with LPS and take a look at particles for twenty-four h. After 24 h, 20 μL of supernatant was transferred from tradition wells to a brand new 96-well plate and 180 μL of Quanti-Blue secreted embryonic alkaline phosphatase (SEAP) answer (Invitrogen) was added. The plate was then incubated for 1-4 h at 37 °C after which level a Synergy microplate reader was used to measure the absorbance of every effectively at 640 nm.
Mouse Nitrogen Mustard Cornea Damage Mannequin
All procedures obtained approval from the Institutional Animal Care and Use Committee (IACUC) at Northwestern College and are adherent to the ARVO Assertion for the Use of Animals in Ophthalmic and Imaginative and prescient Analysis. Feminine C57BL/6 and Balb/c wild-type mice, 6 weeks outdated, had been procured from Charles River. Sulfur mustard (SM) isn’t utilized in a traditional laboratory setting as a result of SM is prone to trigger in depth incapacitating accidents to the eyes, pores and skin, and respiratory tract of uncovered individuals. Whereas NM as it’s a dermatological therapeutic that mimics many if not a lot of the SM etiology. A 0.5% (w/v) answer of mechlorethamine hydrochloride (nitrogen mustard; Sigma) was made in PBS with 3% DMSO instantly earlier than use and stored for not more than 10 min. Mice had been anesthetized by i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). After anesthesia, a sterile filter paper disc (2 mm in diameter) was soaked and saturated with this nitrogen mustard (NM) answer after which positioned on the mouse central corneas for 1 minute. Each eyes had been uncovered to the NM. Previous to publicity, mice had been handled topically with analgesia (proparicaine eye drops). After publicity, to alleviate misery, mice obtained sustained-release buprenorphine dosed at 1 mg/kg SC q48h.
The mice had been euthanized utilizing CO2, adopted by cervical dislocation. After euthanasia, mouse eyes had been eliminated. For histology and immunostaining, eyes had been fastened in formalin. For complete mount, eyeballs had been fastened in PFA. For RNAs, corneal buttons had been dissected and homogenized in Qiazol.
HDL NPs eye drops topical therapy
Two hours postexposure to NM, mice had been topically handled with 2 μM PE-O4 HDL nanoparticle (NP) answer in PBS at a dose of 40 µl per eye or PBS (automobile management). Mice that weren’t uncovered to NM had been used as a non-injured/wholesome management. Mice obtained topical therapies every day for 3 days for the acute irritation mannequin and every day for 14 days to imitate the power, long-term damage mannequin. We didn’t observe any hostile reactions to the topical software of PE-O4 HDL NP, similar to extreme scratching of the eyes, eyelid closure and or exudate across the eyes. Mice tolerated every day software of the NP effectively.
Matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) Pattern Preparation and Knowledge Acquisition
MALDI-MSI pattern preparation and evaluation was carried out on the Built-in Molecular Construction Training and Analysis Heart (IMSERC) at Northwestern College. Recent frozen tissue sections on ITO (indium tin oxide)-coated glass slides had been defrosted underneath vacuum circumstances utilizing a vacuum chamber. After taking an optical picture of the ITO-coat glass slide utilizing a doc scanner, matrix software was performed utilizing a TM-Sprayer (HTX Applied sciences, LLC, Chapel Hill, North Carolina, USA) to spray an answer of 10 mg/mL answer of 9-aminoacridine (9AA) matrix, which was dissolved in 75/25 (v/v) acetonitrile/water. The sprayer settings for 9AA had been: variety of passes, 8; nozzle temperature, 60 °C; circulate charge, 0.12 ml/min; velocity, 1200 mm/min; fuel circulate charge, 3 l/min; and drying time, 10 sec; nebulizer stress, 10 psi; nozzle top, 40 mm; and utilized with a crisscross sample and a spacing of two mm.
MALDI-MSI information acquisition was carried out utilizing a rapifleX MALDI Tissuetyper (Bruker Scientific, LLC, Billerica, Massachusetts, USA). Knowledge was acquired in negative-ion reflectron mode and calibrated utilizing crimson phosphorus earlier than information acquisition. The mass vary collected was m/z 200 – 2000, amassing 200 photographs per pixel, utilizing a laser energy at 75% – 80%, a sampling charge of 1.25 GS/s, and the raster and laser focus had been matched to acquire a ten µm picture decision. The information was processed utilizing SciLS 2023 Professional to generate the ion warmth maps, the place the imaging information had been normalized utilizing the entire ion present (TIC) of the picture dataset.
Medical Imaging for Damage and Corneal Haze
The mouse eyes had been examined for scientific haze scores utilizing a lightweight microscope at designated time intervals following publicity to NM. Corneal haze was graded on a scale of 0 by 4 as follows: 0, no corneal haze; 1, iris element seen; 2, Pupillary margin seen, iris element obscured; 3, pupillary margin not seen; 4, cornea completely opaque. To evaluate epithelial integrity, the ocular surfaces of the mice had been topically stained with 0.5% fluorescein in PBS (10 µL for every eye, then washed with PBS), then noticed underneath cobalt-blue mild on the specified time intervals post-NM publicity.
Actual-Time Quantitative PCR Evaluation
Mouse cornea was meticulously dissected underneath the dissection scope, enabling the isolation of cornea following beforehand printed protocols20,50. Tissues had been homogenized in Qiazol lysis buffer supplied by the miRNeasy mini package (Qiagen, Valencia, CA, USA). RNAs had been extracted from the corneas utilizing the miRNeasy mini package following the producer handbook (Qiagen, Valencia, CA, USA). For RT-qPCR, cDNA was synthesized by the Superscript III reverse-transcription package following the producer handbook (Invitrogen). To quantify gene expression, qPCR was performed in 96 effectively plates with 10 µl response combine on a Lightcycler 96 real-time PCR system (Roche, Indianapolis, IN, US) using a quantitative SYBR inexperienced PCR package (Roche). Particular primers for Mouse Inos, CxCl5, IL1b, Mmp9, CxCl2, and TNFRSF9 (detailed in Supplementary Desk 2) had been utilized. In the meantime, the inner management, Mouse Rpl19, was used for comparative evaluation. The outcomes had been calculated because the fold change in regards to the wild-type unhurt controls. The fold modifications had been decided by ΔΔCt.
Entire mount staining
Entire mount staining was performed as beforehand printed protocols51,52. Briefly, mouse corneas, together with the limbus, had been meticulously dissected underneath microscopic statement. Subsequently, these tissues had been fastened in a formaldehyde fixative answer (FF) for 1 hour and 15 min at 4 °C. The FF answer was ready freshly by mixing 1 ml of 10x PBS, 270 µl of 37% formaldehyde to realize a 1% last focus, 10 µl of two mM MgCl2, 200 µl of 250 mM EGTA (Ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid) (pH 8.0) to achieve 5 mM, 20 µl of 10% NP40 to a last focus of 0.02%, and water to a last quantity of 10 ml. After fixation, the tissues had been washed with 1x PBS containing 0.02% NP40, then fastened in a 4:1 combination of pre-cooled (to −20 °C) methanol and DMSO for two h. Then, the eyes had been transferred to pre-cooled 100% methanol, wherein they may very well be saved at −20 °C for a number of weeks. Following fixation, tissues had been stained with both rat anti-CD31 (BD Biosciences®) or mouse anti-muc5 (Thermo Fisher Scientific®) at a dilution of 1:1000. DAPI was used for counterstaining at a 1:2000 dilution. To make sure the corneas lay as flat as doable on glass slides, small incisions had been made on the edges earlier than placement. Entire-mount imaging was carried out utilizing a Nikon CSU-W1 Yokogawa Sora Confocal Microscope, geared up with a ten× goal lens. Imaging was performed within the z-plane, capturing 10 sequential sections to make sure three-dimensional visualization.
Histology
Mouse complete eyes had been dissected after which fastened in a impartial buffered 10% formalin answer for twenty-four hours. Tissues had been embedded in paraffin, sectioned, and stained utilizing hematoxylin and eosin. Briefly, after deparaffinization and re-hydration, slides had been stained in Mayer’s Hematoxylin Resolution for 30 sec and rinsed in operating faucet water for two min. Slides had been dipped in 1% Acid Alcohol twice and washed with operating faucet water for two min. Slides had been counterstained in Eosin Resolution (1 ml Eosin Resolution + 2ul Acetic Acid Glacial) for 2-3 min. After rinsing with 3-4 washes of faucet water, slides had been dehydrated and mounted. The ensuing slides had been analyzed underneath a Zeiss Axioplan 2 Gentle Microscope. Epithelial thickness and infiltrated immune cell quantification inside the stroma had been analyzed with ImageJ software program.
Statistical Evaluation
Visible graphics had been generated, and statistical analyses had been performed utilizing GraphPad Prism 10.2.0 software program (San Diego, CA). Statistical significance was assessed with an unpaired t-test, with information offered as means±customary error of the imply (SEM). Haze rating was thought-about as categorical information and a Mann−Whitney U take a look at was performed. A p worth of




