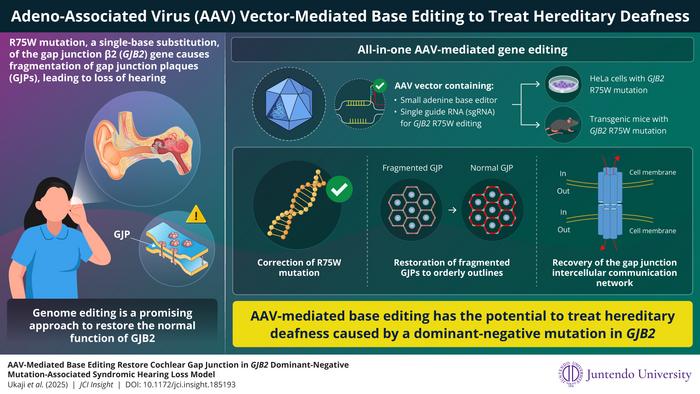

In groundbreaking advances within the area of genetic medication, researchers from Japan have pioneered a revolutionary gene remedy concentrating on syndromic listening to loss brought on by a mutation within the GJB2 gene. This progressive method seeks to deal with the urgent want for healing therapies which might be at the moment absent for a big proportion of sufferers affected by genetic listening to loss. By using a sophisticated gene-editing know-how, researchers goal to restore the R75W mutation—a dominant-negative variant of the GJB2 gene liable for a considerable variety of hereditary deafness instances. The work, led by Affiliate Professor Dr. Kazusaku Kamiya and his expert staff at Juntendo College, is ready to vary the panorama of genetic therapies for auditory issues.
Congenital listening to loss presents a big problem, affecting hundreds of thousands worldwide. Estimates recommend that genetic components are liable for roughly half of all situations of hereditary listening to loss, with the GJB2 gene being a pivotal participant. Encoding the protein Connexin 26 (CX26), GJB2 types essential intercellular hole junctions that facilitate the change of ions and signaling molecules between cells, notably within the delicate construction of the cochlea. The disturbance within the operate of those hole junctions on account of mutations can result in extreme auditory impairments.
The implications of GJB2 mutations are profound and sophisticated. Usually, recessive mutations might be addressed by means of gene substitute therapies. Nevertheless, dominant-negative mutations, like R75W, pose a extra formidable problem as they not solely disrupt the operate of the mutated gene but additionally inhibit the traditional operate of the wild-type gene. This attribute necessitates the event of exact gene modifying capabilities to successfully restore auditory operate and structural integrity.
With this background, the analysis staff from Juntendo College launched into a mission to create a viable gene remedy answer for people carrying the R75W mutation. Their preliminary findings highlighted the shortage of current therapeutic interventions for this situation, emphasizing the pressing want for progressive approaches to deal with hereditary listening to loss. Of their quest, the researchers developed a tailor-made adeno-associated virus vector (AAV-Sia6e), which possesses an enhanced capability for concentrating on and coming into the specialised interior ear cells, often known as hair cells, pivotal for listening to.
One pivotal side of their analysis was the miniaturization of the bottom modifying instrument, which encompassed the dimerization of SaCas9-NNG and ABE8e (adenine base editor). This progressive method circumvents the dimensions limitations inherent to traditional gene supply programs. By streamlining the gene-editing instrument, the staff ensured its compatibility with the AAV vector they engineered. This seamless integration not solely broadened the therapeutic software but additionally optimized the effectivity of the gene-editing course of.
Their complete methodology seamlessly led to the profitable uptaking and execution of exact genetic modifications in cultured human cells exhibiting the GJB2 R75W mutation. The on-target modifying resulted in a selected T-to-C conversion that restored regular hole junction formation, thereby reinstating the physiological performance of intercellular communication—a crucial element in sustaining auditory well being. This exceptional final result underscores the utility of AAV-mediated genome modifying as a viable route for addressing genetic types of listening to impairments.
To validate their speculation additional, the analysis staff took their research to the following stage by using a transgenic mouse mannequin particularly developed to exhibit the GJB2 R75W mutation. By means of the analysis of cochlear samples post-AAV-mediated modifying, vital enhancements have been famous. The distinct formation of hole junctions within the transgenic mice mimicked the buildings present in wild-type specimens, illustrating the precision and efficacy of their modifying technique.
Dr. Kamiya elucidated the broader implications of their analysis, indicating that their all-in-one AAV vector may pave the best way for therapeutic developments not solely in listening to loss related to GJB2 but additionally in different genetic issues involving hole junctions. The potential to develop cost-effective and broadly relevant gene therapies alerts a transformative period in treating genetic listening to loss, providing hope to numerous people craving for efficient interventions.
Furthermore, the researchers highlighted the anticipated security benefits of their method over current CRISPR-Cas9 gene-editing applied sciences. The ABE-based methodology is projected to yield decrease cytotoxicity charges, contributing to a safer therapy paradigm for sufferers. With security and effectivity prioritized, the implications of this analysis prolong past addressing hereditary listening to loss; they open avenues for exploring genetic therapies throughout a wider spectrum of circumstances.
By means of vital developments in gene-editing applied sciences, these findings stand to reshape the therapeutic panorama for hereditary deafness. Because the incidence of genetic listening to loss rises, the crucial nature of such analysis turns into much more obvious. The potential for future variations of their AAV-mediated base modifying know-how to focus on different mutations inflicting auditory impairments reinforces the urgency and relevance of this work.
In abstract, the dedication and progressive spirit exhibited by Dr. Kamiya and his analysis staff replicate a dedication to advancing the frontiers of genetic remedy. Their analysis elucidates a pathway in the direction of efficient interventions that goal to revive listening to and enhance the standard of life for 1000’s bothered by genetic auditory issues. The promise of AAV-mediated genome modifying resonates far past the realm of listening to loss, heralding a future the place genetic illnesses could also be met with efficient and accessible treatments.
This groundbreaking analysis was printed within the esteemed journal JCI Perception, underscoring its significance and potential affect throughout the scientific neighborhood. As the sector of genetic medication continues to push the boundaries of chance, developments like these offered by Dr. Kamiya’s staff illuminate the way forward for therapeutic by means of science.
Topic of Analysis: Animals
Article Title: AAV-mediated base modifying restores cochlear hole junction in GJB2 dominant-negative mutation-associated syndromic listening to loss mannequin
Information Publication Date: 10-Mar-2025
Internet References: https://doi.org/10.1172/jci.perception.185193
References:
Picture Credit: Credit score: Dr. Kazusaku Kamiya from Juntendo College, Japan
Key phrases
Tags: superior gene-editing technologyauditory issues genetic therapiesConnexin 26 and hearingcurative therapies for listening to impairmentDr. Kazusaku Kamiya researchgene remedy for listening to lossgenome modifying for genetic deafnessGJB2 gene mutation treatmenthereditary deafness genetic factorsinnovative approaches to congenital listening to lossJuntendo College genetic researchR75W mutation in GJB2




