Assortment of Spatial Transcriptomics Information from Human Fallopian Tube Epithelium
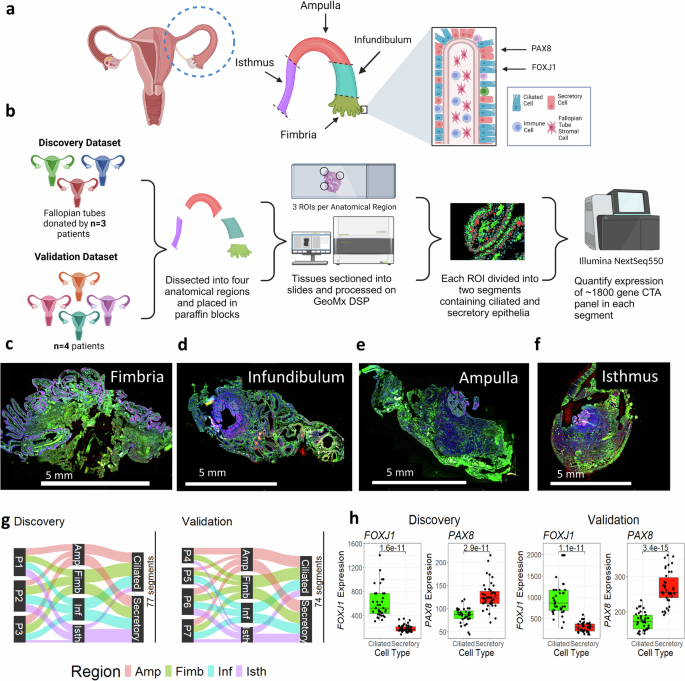
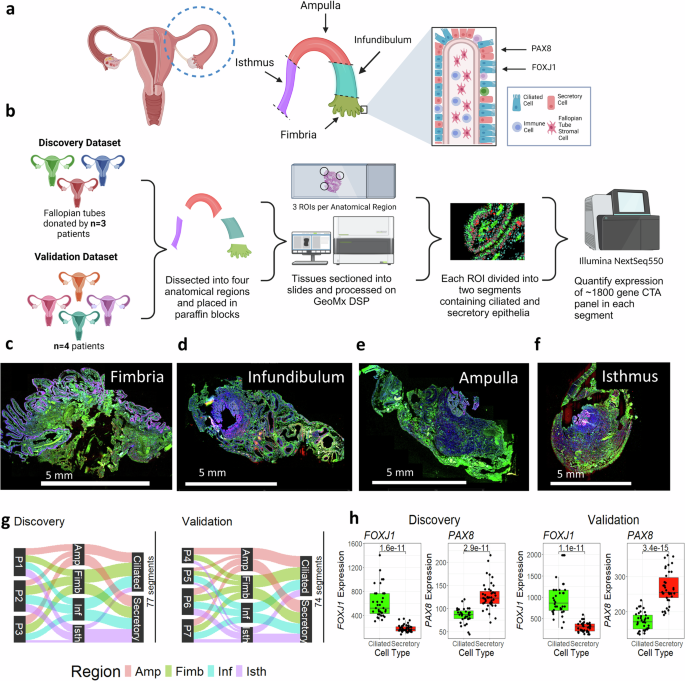
To profile the fallopian tube alongside the proximal to distal axis, we collected seven tissue specimens from premenopausal sufferers (age 25-48, imply 39) present process salpingectomy for non-cancer indications unrelated to fallopian tube well being. Affected person traits are detailed in Supplementary Desk 1. Samples have been divided into 4 anatomical areas (isthmus, ampulla, infundibulum, and fimbria) (Fig. 1a). We used immunofluorescence to tell apart secretory (PAX8) and ciliated (FOXJ1) epithelial cells and chosen consultant segments in every anatomical area (Fig. 1b), as beforehand described36. Transcriptomes have been profiled utilizing the GeoMx Most cancers Transcriptome Atlas, protecting ~1800 cancer-related and cell marker transcripts. Consultant areas from Affected person 2 are proven in Fig. 1c–f.
a Anatomy of the fallopian tube, displaying the 4 main anatomical areas and main cell varieties. The transcription components PAX8 and FOXJ1 are used to tell apart secretory and ciliated cells. b Experimental workflow, displaying creation of discovery (n = 3) and validation (n = 4) cohorts. Following dissection into anatomical areas, areas of curiosity (ROIs) containing epithelial cells have been chosen, segmented by cell kind, and probe barcodes have been collected from every section. Barcodes have been sequenced on the Illumina NextSeq550, and the ultimate output was transcript abundance for all transcripts within the ~1800-gene CTA panel. c–f Consultant scans of the fimbria, infundibulum, ampulla, and isthmus from affected person 2, displaying staining and ROI choice. Nuclei (blue) = SYTO13; FOXJ1 expressing ciliated cells (inexperienced) = CY3, PAX8 expressing secretory cells (pink) = AF594; Scale bar = 5 mm. g A Sankey diagram displaying distribution of segments within the discovery and validation datasets throughout three classes: affected person of origin, anatomical area (Amp = Ampulla (pink), Fim = Fimbria (inexperienced), Inf = Infundibulum (blue), Isth = Isthmus (purple)), and cell kind (ciliated or secretory). A complete of 77 segments (out of 110 initially collected) stay following high quality management. h Boxplot displaying expression of FOXJ1 and PAX8 in ciliated and secretory segments for discovery (n = 77 segments) and validation (n = 74 segments) datasets. Ciliated = inexperienced, Secretory = pink; Decrease and higher hinges of boxplot correspond to first (Q1) and third quartiles (Q3); central line corresponds to median. Whiskers of boxplot vary from Q3 + 1.5*IQR to Q1-1.5*IQR. P-values calculated utilizing two-sided t-test. Created in BioRender. Sipes, J. (2025) https://BioRender.com/a22y908.
For validation, the dataset was break up into discovery (n = 3) and validation (n = 4) cohorts (Fig. 1a). Of 110 segments within the discovery cohort, 77 handed high quality management; 74 of 94 segments handed within the validation cohort (Supplementary Desk 2). High quality management and quantile normalization37 have been carried out individually for each cohorts. After low abundance transcripts (detected in 1g.
Segmentation validation confirmed anticipated marker expression: FOXJ1 was greater in ciliated segments (Discovery logFC: 1.73, p = 1.6 × 10-11; Validation logFC: 1.71, p = 1.1 × 10-11), whereas PAX8 was greater in secretory segments (Discovery logFC: -0.58, p = 2.91 × 10-11, Validation logFC: -0.62, p = 3.4 × 10-15) (Fig. 1h). These outcomes verify profitable segmentation of ciliated and secretory populations, enabling downstream evaluation.
To evaluate menstrual cycle standing, OVGP1 immunostaining was carried out. OVGP1, a fallopian tube-specific protein concerned in enhancing sperm capacitation, fertilization price, and embryo improvement38, is extremely differentially expressed within the fallopian tube through the menstrual cycle34,39,40, and can be utilized as a marker of menstrual cycle standing33. OVGP1 expression peaks throughout late follicular part (pre-ovulatory), and reduces in luteal part (post-ovulatory)34,39,40. Comparable OVGP1 upregulation is noticed throughout estrus (late follicular stage) in different mammalian oviducts, together with sheep41,42, cow43, goat44, cat45, pig46, baboon47, and canine48 oviducts, in addition to sheep oviductal fluid49. Consultant slides of all areas and sufferers have been stained for OVGP1, summarized in Supplementary fig. 1. IHC staining signifies Sufferers 2 and three have been in late follicular part (100% epithelial cells OVGP1 + ), whereas Sufferers 4 and 5 have been in luteal part ( 1A-C). One affected person (Affected person 6) had a earlier sterilization utilizing a Filshie clip to bilaterally constrict the FT, mirrored within the OVGP1 standing. Distal segments (fimbria and infundibulum) remained OVGP1 + , in line with ovarian stimulation whereas proximal segments (ampulla and isthmus) have been OVGP1-, reflecting lack of ovarian signaling brought on by the constriction.
Transcriptomic profiling of FTE exhibits spatially regulated transcripts
The invention cohort was analyzed to determine differentially expressed genes (DEGs) between areas. Pairwise comparisons have been carried out evaluating ciliated and secretory cells in every anatomical area to their counterparts in all different areas to determine DEGs. Supplementary fig. 3-4 exhibits DEGs (p-value 2(FC) | > 0.5) in all pairwise comparisons within the discovery and validation cohorts.
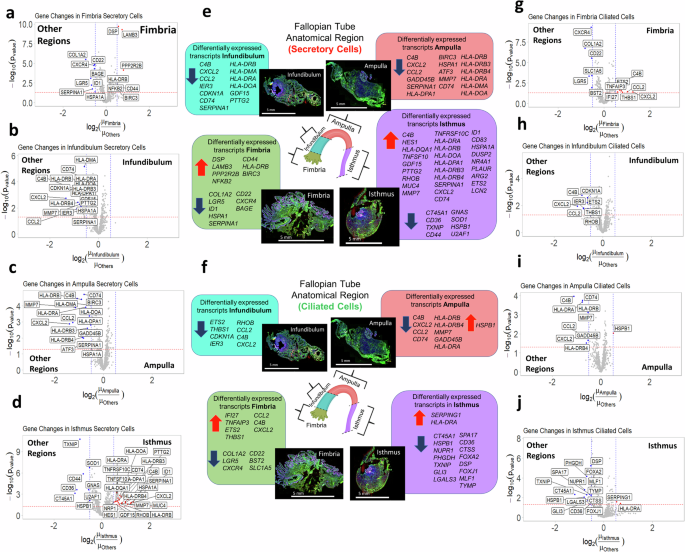
To pinpoint region-specific DEGs, we in contrast the typical expression in every area to all different areas. Determine 2a-d exhibits volcano plots for the invention cohort with DEGs in secretory cells of the fimbria, infundibulum, ampulla, and isthmus, with key genes summarized in Fig. 2e. Outcomes for ciliated cells are proven in Fig. 2f-j, and equal validation cohort plots are supplied in Supplementary fig. 5.
Volcano plots for secretory cell segments displaying transcripts differentially expressed in (a) the fimbria, (b) the infundibulum, (c) the ampulla, and (d) the isthmus relative to all different areas. e) A abstract of all transcripts noticed upregulated and downregulated within the fallopian tube. Scale bar = 5 mm f) A abstract of all transcripts noticed upregulated and downregulated in ciliated cells. Scale bar = 5 mm g-j) Volcano plots for ciliated cell segments displaying transcripts differentially expressed in (g) the fimbria, (h) the infundibulum, (i) the ampulla, and (j) the isthmus relative to throughout areas. Purple factors: upregulated transcripts; blue factors: downregulated transcripts, pink traces: p-value cutoff (({-{{mathrm{log}}}}_{10}(p)=1.3)); blue traces = FC cutoff (({{{mathrm{log}}}}_{2}({FC}),=,+/-,0.5)). Created in BioRender. Sipes, J. (2025) https://BioRender.com/p37o480.
We recognized transcripts with sequential expression alongside the proximal to distal axis. Transcripts have been categorized as rising (greater in the direction of the fimbria) or reducing (greater in the direction of the isthmus) throughout areas. These DEGs are listed in Supplementary Tables 3–6.
We carried out Gene Ontology evaluation on DEGs recognized by pairwise comparability throughout fallopian tube areas (Supplementary fig. 2). In secretory cells, genes differentially expressed within the fimbria versus isthmus have been associated to tissue improvement (nGenes = 15, FDR = 5.48 × 10^-8), epithelium improvement (nGenes = 12, FDR = 6.00 × 10^-5), apoptotic course of (nGenes = 12, FDR = 0.004), and cell loss of life (nGenes = 13, FDR = 0.003). Moreover, genes concerned within the response to oxygen-containing compounds have been differentially expressed between the infundibulum and isthmus (Supplementary fig. 2e). Immune-related pathways, together with cytokine signaling (fimbria versus ampulla) and lymphocyte proliferation (fimbria versus infundibulum), have been considerably enriched.
Since repeated ROS publicity from follicular fluid is hypothesized to contribute to early HGSOC improvement25, an understanding of fallopian tube response to ROS and regulation of apoptosis might present vital details about the early improvement of ovarian most cancers. Based mostly on these findings, we centered on DEGs of distinct fallopian tube cell varieties, in addition to transcripts associated to ROS and immune response. To additional examine mobile differentiation throughout the FT, we analyzed markers of mature ciliated and secretory cells throughout anatomical areas.
Markers of mature ciliated cells present elevated expression within the distal FTE
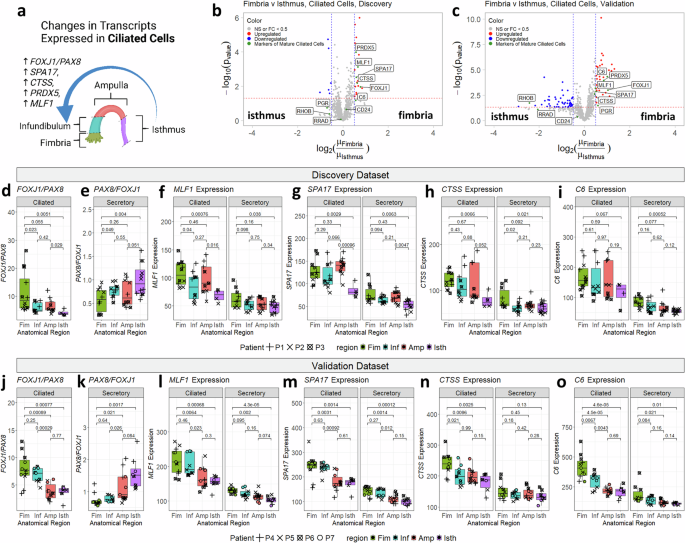
Within the discovery cohort, fallopian tube cell kind markers (FOXJ1, PAX8) confirmed anatomical regulation (Fig. 3a): FOXJ1 expression was greater in ciliated cells approaching the fimbria (p = 0.011), whereas PAX8 was decrease (p = 0.0065; Supplementary fig. 5A-B). These transcription components management cell-differentiation within the FT epithelium; through the secretory-to-ciliated transition, PAX8 is downregulated whereas FOXJ1 is upregulated, with immature transitional ciliated cells coexpressing each markers31. We used a set of mature ciliated cell markers recognized by single-cell sequencing31 and in contrast expression within the fimbria and isthmus (Fig. 3b).
a Diagram displaying differential expression of markers of mature ciliated cells alongside the fallopian tube. Volcano plots evaluating expression of transcripts between fimbria and isthmus sections within the discovery (b) and validation (c) cohorts, with mature ciliated cell markers31 highlighted in inexperienced. Purple traces: p-value cutoff (({-{{mathrm{log}}}}_{10}(p)=1.3)); blue traces = FC cutoff (({{{mathrm{log}}}}_{2}({FC}),=,+/-,0.5)). All p-values are calculated utilizing t-test.) d Boxplot displaying ratio of FOXJ1:PAX8 ratio in ciliated cells (discovery cohort, n = 35). e Boxplot displaying PAX8:FOXJ1 ratio in secretory cells (discovery cohort, n = 42). Boxplots displaying expression of mature ciliated cell markers MLF1 (f), SPA17 (g), CTSS (h), and C6 (i) in ciliated and secretory segments (discovery cohort). j-o Boxplots plotting the identical ratios or gene expression modifications as (d-i), however for the validation cohort (n = 36 ciliated and n = 38 secretory segments). Respectively, PAX8:FOXJ1 ratio in ciliated cells (j), PAX8:FOXJ1 ratio in secretory cells (okay), MLF1 expression (l), SPA17 expression (m), CTSS expression (n), and C6 expression (o). Decrease and higher hinges of boxplot correspond to first (Q1) and third quartiles (Q3); central line corresponds to median. Whiskers of all boxplots vary from Q3 + 1.5×IQR to Q1-1.5×IQR. All p-values calculated utilizing two-sided t-test. Created in BioRender. Sipes, J. (2025) https://BioRender.com/p37o480.
Moreover, we performed a sequential evaluation alongside the proximal to distal axis, evaluating the expression in the fimbria versus infundibulum, infundibulum versus ampulla, and ampulla versus isthmus (Supplementary fig. 6C-E). All comparisons, aside from the infundibulum versus ampulla comparability (which had comparable expression patterns), revealed elevated expression of mature ciliated cell markers in the distal section approaching the fimbria.
FOXJ1/PAX8 expression ratios in ciliated cells (Fig. 3d) elevated (p = 0.0051) approaching the fimbria, whereas PAX8/FOXJ1 ratios in secretory cells (Fig. 3e) have been greater approaching the isthmus (p = 0.004). This discovering means that extra mature ciliated cells and transitional secretory cells are discovered within the fimbria in comparison with the isthmus. Extra mature ciliated cell markers, together with ciliogenesis-related50 MLF1 (p = 0.00076), SPA17 (p = 0.0029), CTSS (p = 0.0066), and C6 (p = 0.067) additionally elevated close to the fimbria, with weaker tendencies in secretory cells (Fig. 3f–i).
The validation cohort confirmed these outcomes. As soon as once more, markers of mature ciliated cells have been enriched within the fimbria in comparison with the isthmus (Fig. 3c). Within the validation dataset, FOXJ1 expression as soon as once more elevated approaching the fimbria (Supplementary fig. 6B). Ratios of FOXJ1/PAX8 in ciliated cells, PAX8/FOXJ1 in secretory cells, and expression of MLF1, SPA17, CTSS, and C6 have been all elevated approaching the fimbria (Fig. 3j-o). These findings display constant enrichment of mature ciliated cells markers towards the distal fallopian tube.
Genes concerned in cell-cell adhesion are differentially expressed alongside the fallopian tube axis
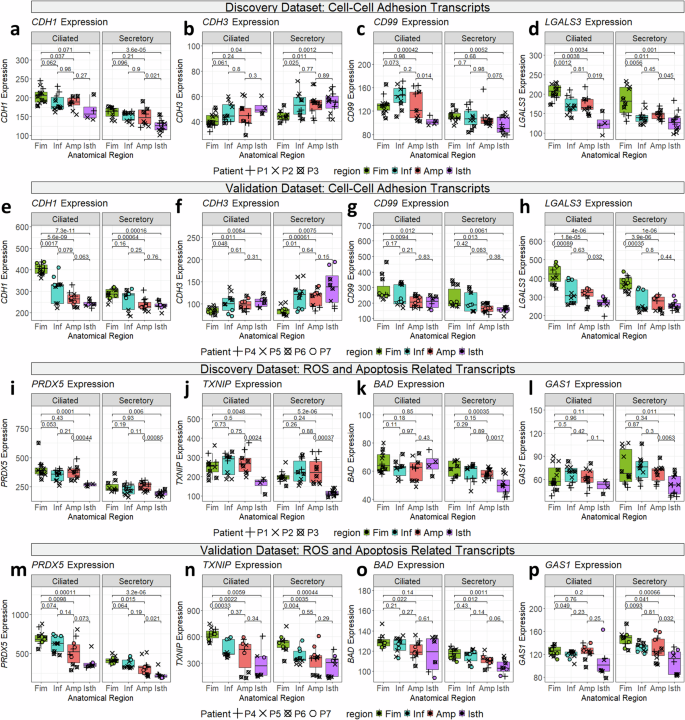
Cell-cell adhesion genes (CDH1, CDH3, LGALS3, and CD99) are differentially expressed throughout the fallopian tube. CDH1 (E-cadherin), a tumor suppressor gene regularly downregulated in most cancers51, elevated distally, peaking within the fimbria (Fig. 4a). Against this, CDH3 (P-cadherin) exhibits the other sample, with greater expression approaching the isthmus (Fig. 4b), maybe reflecting a change in cell-ECM interactions alongside the fallopian tube. (It must be famous that even within the isthmus, CDH3 transcripts nonetheless have decrease total expression in comparison with CDH1 transcripts). Two different transcripts concerned in cell-cell adhesion confirmed differential expression: LGALS3 (Galectin-3) and CD99 (MIC2) enhance towards the distal fallopian tube in each secretory and ciliated cells (Fig. 4c, d). The validation cohort confirmed these tendencies, displaying comparable patterns of adhesion gene regulation alongside the distal to proximal axis (Fig. 4e-h).
a–p Boxplots of transcripts differentially expressed in numerous anatomical areas of the fallopian tube, displaying expression in each ciliated and secretory segments. Level shapes are chosen based mostly on affected person info. P-values for chosen comparisons are plotted. All p-values are calculated utilizing two-sided t-test. inexperienced: fimbria; blue: infundibulum, pink: ampulla, purple: isthmus; circles: pt_1, squares: pt_2, diamonds: pt_3, Decrease and higher hinges of boxplot correspond to first (Q1) and third quartiles (Q3); central line corresponds to median. Whiskers of boxplot vary from Q3 + 1.5*IQR to Q1-1.5*IQR. Plots (a–h) present cell-cell adhesion transcripts expressed within the fallopian tube epithelium within the discovery (n = 77) (a–d) and validation cohorts (n = 74) (e–h). Plots (i–p) present transcripts related to reactive oxygen species or apoptosis/cell cycle expressed within the fallopian tube epithelium within the discovery (i–l) and validation cohorts (m–p).
Genes concerned in ROS and apoptosis are upregulated within the distal FTE
Genes concerned in oxidative stress response (TXNIP, PRDX5) and apoptosis (BAD, GAS1) present distinct spatial expression within the fallopian tube. PRDX5, encoding the antioxidant enzyme Peroxiredoxin-5, is upregulated in ciliated cells close to the fimbria, with an analogous however weaker development noticed in secretory cells (Fig. 4i). TXNIP, encoding Thioredoxin Interacting Protein, is considerably downregulated within the isthmus throughout each cell varieties (Fig. 4j).
Apoptosis-related genes additionally exhibit spatial regulation. BAD (BCL2-associated agonist of cell loss of life) is a promoter of cell loss of life52 which is upregulated approaching the fimbria in secretory, however not ciliated cells (Fig. 4k). Equally, GAS1 (Development Arrest Particular 1), which promotes cell cycle arrest and apoptosis, is upregulated in secretory cells approaching the fimbria (Fig. 4l).
The validation cohort corroborated these findings for PRDX5 and TXNIP, revealing a constant sample of diminished expression within the isthmus relative to the fimbria (Fig. 4m-n). Nevertheless, whereas the invention dataset confirmed equal expression of those genes within the fimbria, infundibulum, and ampulla, the validation dataset demonstrated a linear enhance towards the fimbria. Notably, the validation samples have been largely in luteal part, as indicated by OVGP1 staining, so this probably displays an adaptive response to ROS publicity from follicular fluid. Comparable tendencies have been noticed for BAD and GAS1, confirming their fimbrial upregulation in secretory cells (Fig. 4o-p). These findings counsel segment-specific regulation of oxidative stress and apoptotic pathways in response to environmental pressures, comparable to follicular fluid publicity.
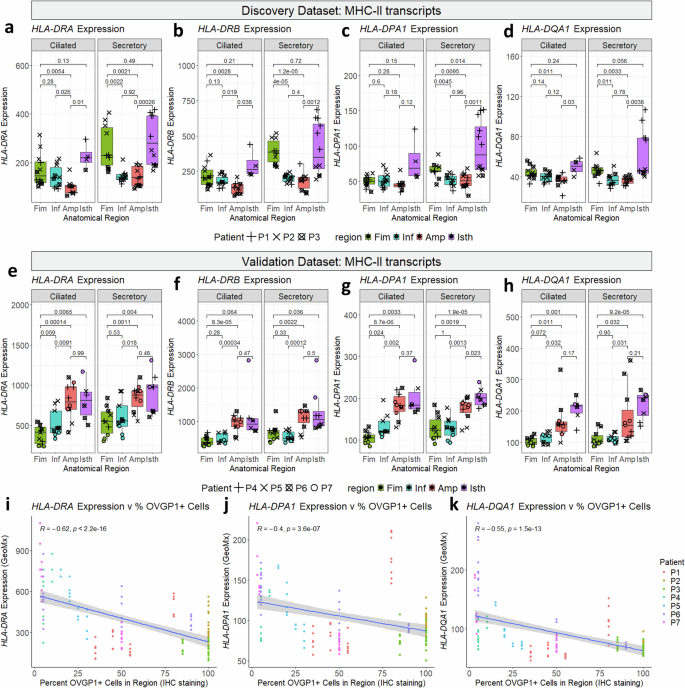
MHC-II transcripts are downregulated within the inside fallopian tube throughout follicular part and inversely correlated with OVGP1 expression
Within the discovery cohort, MHC-II transcripts present among the highest expression modifications, with elevated expression within the fimbria and isthmus in comparison with the ampulla and infundibulum. Though this impact was largest in secretory cells, ciliated cells present an analogous development. All three MHC-II isotypes (HLA-DR, HLA-DP, HLA-DQ) confirmed comparable expression modifications. For instance, HLA-DR subunits DRα and DRβ (encoded by HLA-DRA and HLA-DRB), present greater expression in secretory cells of the isthmus and fimbria (Fig. 5a, b). Different HLA-DR isoforms (HLA-DRB3, HLA-DRB4; Supplementary fig. 7A-B), the HLA-DPα subunit (HLA-DPA1, Fig. 5c), and the HLA-DQα subunit (HLA-DQA1, Fig. 5d) confirmed the identical sample, whereas HLA-DQB1 was solely considerably overexpressed within the isthmus (Supplementary fig. 7C). Of those, HLA-DR transcripts (the main MHC-II isotypes) show the very best expression and the most important regional variations.
a–d Boxplots of MHCII transcripts differentially expressed within the discovery cohort (n = 77 segments). a HLA-DRA, b HLA-DRB, c HLA-DPA1, d HLA-DQA1. e-h) Boxplots of MHCII transcripts differentially expressed within the validation cohort (n = 74 segments): e HLA-DRA, f HLA-DRB, g HLA-DPA1, h HLA-DQA1. All p-values are calculated utilizing two-sided t-test. Inexperienced: fimbria; blue: infundibulum, pink: ampulla, purple: isthmus; circles: pt_1, squares: pt_2, diamonds: pt_3, Decrease and higher hinges of boxplot correspond to first (Q1) and third quartiles (Q3); central line corresponds to median. Whiskers of boxplot vary from Q3 + 1.5 × IQR to Q1 – 1.5 × IQR. Scatterplots evaluating i HLA-DRA, j HLA-DPA1, or okay HLA-DQA1 expression to proportion of OVGP1 constructive cells in equal IHC stained slides. Pearson’s correlation coefficient (R) and related p-value are proven on scatterplots (calculated utilizing the R package deal ‘ggpubr‘). Gray border across the line of finest match exhibits the 95% confidence interval.
Genes concerned in MHC-II peptide loading, together with HLA-DMA, HLA-DOA, and CD74, present the identical sample, whereas associated transcript HLA-DMB was solely upregulated within the fimbria (Supplementary fig. 7D-G). Notably, the HLA locus has complement genes C2, C4B, and CFB situated between the MHC-II and MHC-I loci, of which two genes (C4B and CFB) have been additionally upregulated in these areas (Supplementary fig. 7H-I). Collectively, these findings point out region-specific upregulation of MHC-II transcripts on the FT’s ends and downregulation in its inside.
Surprisingly, the validation dataset confirmed that each one of those MHC-II genes have been upregulated solely within the isthmus, slightly than the isthmus and the fimbria. This development utilized to all MHC-II isotypes (HLA-DR, -DP, -DQ; Fig. 5e-h, Supplementary fig. 7J-M), their helper genes (HLA-DMA, -DMB, -DOA, and CD74; Supplementary fig. 7N-Q), and complement components (C2, C4, CFB; Supplementary fig. 7R-T).
We suspected this sudden shift was attributable to variations in MHC-II expression through the menstrual cycle. To research this risk, we in contrast expression of MHC-II genes (HLA-DRA, HLA-DPA1, and HLA-DQA1) to OVGP1 standing as decided by IHC staining (Fig. 5i-k). We discovered a powerful damaging correlation between OVGP1 expression and MHC-II expression (HLA-DRA: R = -0.62, p × 10^-16, HLA-DPA1: R = -0.4, p = 3.6 × 10^-7, HLA-DQA1: R = -0.55, p = 1.5 × 10^-13). These outcomes counsel that MHC-II is downregulated within the follicular part (when OVGP1 is highest) and upregulated within the luteal part.
To verify this, we reanalyzed prior FT transcriptomics research31,34. Laser seize microdissection information (GEO: GSE129348) revealed OVGP1 was upregulated within the follicular part, whereas MHC-II transcripts have been upregulated within the luteal part (Supplementary fig. 8A). MHC-II transcripts confirmed damaging correlation with OVGP1 expression (Supplementary fig. 8B). Single-cell sequencing information (GEO: GSE178101) corroborated these findings, displaying that OVGP1 excessive cells have low MHC-II expression, and MHC-II transcripts negatively correlate with OVGP1 expression (Supplementary fig. 8C-D). Extra just lately revealed single-cell sequencing work by Weigert et al. goes additional, displaying that secretory cells of the fallopian tube cluster into three classes – one group (recognized as SE1 by the authors) has excessive expression of MHC-II genes, whereas two different teams (SE2 and SE3) have excessive expression of OVGP133. The authors present that the OVGP1 expressing SE2 cells are extra frequent pre-ovulation (follicular part), whereas the MHC-II expressing SE1 cells are extra frequent post-ovulation (luteal part), offering additional affirmation of menstrual cycle particular results33. These findings counsel that MHC-II expression within the fallopian tube is cyclically regulated, with decrease expression within the follicular part when OVGP1 is ample, seemingly attributable to modifications in MHC-II expression in OVGP1-producing secretory cells.
Markers of OVGP1 producing cells are upregulated within the inside of the FT through the follicular part
Evaluation of single-cell sequencing information recommended downregulation of MHC-II within the follicular part is linked to OVGP1-producing secretory cells, recognized as peg cells by Ulrich et al.31. Utilizing peg cell markers recognized on this dataset31, we in contrast expression throughout fallopian tube areas. Peg cell markers have been persistently enriched approaching the ampulla, with highest expression within the inside areas (ampulla and infundibulum) and decrease expression within the fimbria and isthmus (Supplementary fig. 9A-C). Whereas our panel doesn’t embody OVGP1, different markers of peg cells, together with LGR5, NOTCH2, COL1A2, and SERINC5, present elevated expression in secretory cells approaching the ampulla and a slight lower in expression for many samples within the isthmus (Supplementary fig. 9D-G).
Nevertheless, these tendencies weren’t replicated within the validation dataset. Whereas NOTCH2 confirmed an analogous however statistically insignificant development, LGR5 and COL1A2 displayed no clear development, whereas SERINC5 confirmed elevated expression within the fimbria (Supplementary fig. 9H-Ok). The validation cohort had low OVGP1 immunohistochemistry expression, maybe explaining why peg cell markers now not show the identical tendencies. These findings counsel OVGP1+ peg cells are enriched within the ampulla and infundibulum throughout follicular part (when OVGP1 manufacturing peaks), and return to decrease ranges throughout luteal part.
Hormone receptor expression throughout the fallopian tube varies with menstrual cycle standing and OVGP1 expression
Steroid hormone receptors are essential to fallopian tube operate53, influencing organogenesis and regulating gene expression. Their exercise is aided by pioneer components, which work together with condensed chromatin to reinforce accessibility for hormone receptor binding54. Within the discovery cohort, ESR1 (estrogen receptor α) was upregulated approaching the isthmus in secretory and ciliated cells (Supplementary fig. 10A). In distinction, PGR (Progesterone Receptor) and AR (Androgen Receptor) didn’t present vital variations (Supplementary fig. 10B-C). Pioneer components implicated in ESR1 signaling additionally diverse: PBX1 expression elevated approaching the isthmus in each cell varieties, whereas FOXA2 was upregulated within the fimbria, however just for ciliated cells (Supplementary fig. 10D-E).
Within the validation cohort, gene expression patterns differed, seemingly reflecting variations in menstrual cycle standing. ESR1 expression remained uniform throughout the fallopian tube as an alternative of accelerating towards the isthmus (Supplementary fig. 10F). PGR expression elevated towards the fimbria in each cell varieties (Supplementary fig. 10G), whereas AR continued to point out no development (Supplementary fig. 10H). Apparently, FOXA2 maintained greater expression close to the fimbria in each cohorts (Supplementary fig. 10I), however PBX1 expression was inconsistent (Supplementary fig. 10J). A comparability of transcript expression with OVGP1 staining confirmed a powerful constructive correlation with PGR expression (R = 0.48, p = 3.6*10^-10), and a weak, however vital correlation with ESR1 (R = 0.21, p = 0.01) (Supplementary fig. 10K-L). These findings align with reviews that OVGP1+ cells specific hormone receptors, notably PGR31. Collectively, these outcomes counsel dynamic regulation of hormone receptors and pioneer components within the fallopian tube, pushed by spatial location and menstrual cycle standing.