
In a groundbreaking research printed in Science Bulletin, researchers have unveiled the essential function of the helicase DHX36 in sustaining chromatin structure and making certain correct ribosomal RNA (rRNA) processing throughout mouse oocyte growth. This discovery sheds new gentle on the molecular underpinnings of feminine fertility and early embryogenesis, highlighting DHX36’s important operate in resolving G-quadruplex (G4) constructions that affect chromatin accessibility and gene expression. The implications of this analysis resonate deeply throughout the fields of molecular biology and reproductive science, opening avenues for higher understanding infertility linked to chromatin dysregulation.
G-quadruplex constructions, fashioned by guanine-rich DNA or RNA sequences by Hoogsteen hydrogen bonding, symbolize distinctive secondary configurations that may profoundly have an effect on nucleic acid metabolism. DHX36, often known as RHAU, is a specialised helicase recognized for its capability to unwind G4 constructions, thus modulating transcriptional and post-transcriptional processes. Whereas DHX36’s involvement in processes similar to spermatogenesis and cardiac growth is nicely documented, its particular function in oocyte maturation and early embryonic growth has remained enigmatic—till now.
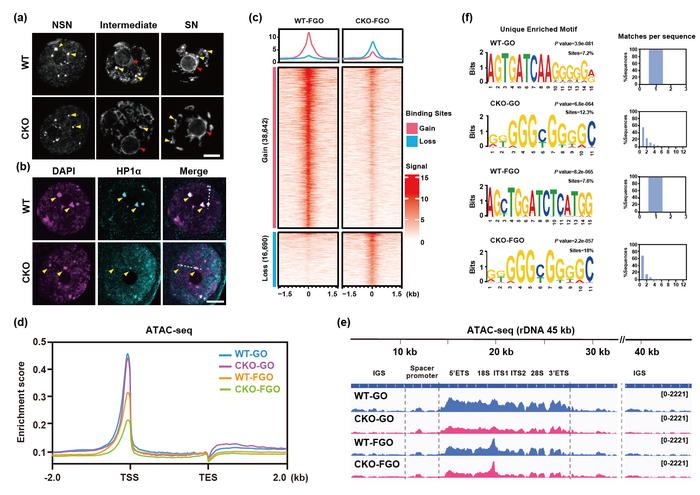
The analysis crew launched into a complete investigation into the expression and localization patterns of DHX36 inside mouse oocytes. They noticed strong DHX36 expression in ovarian tissue, predominantly concentrated within the nucleus. Intriguingly, as oocytes progressed by developmental levels, the spatial distribution of DHX36 shifted from the nucleoplasm to the nucleolus, concomitant with nucleolar conformational adjustments. This dynamic localization hinted at a possible regulatory function linked intimately with nucleolar operate and chromatin group.
To discover DHX36’s practical impression, investigators engineered an oocyte-specific conditional knockout (CKO) mouse mannequin using the Cre-LoxP recombination system. The phenotypic penalties in DHX36-deficient females have been hanging: full infertility, characterised by profound hormonal irregularities and impaired ovulatory responses. This phenotype underscored DHX36’s indispensable function in reproductive biology and prompted deeper mechanistic inquiries.
Detailed phenotypic analyses revealed multifaceted meiotic and post-fertilization defects in DHX36-CKO oocytes. Primarily, the vast majority of oocytes did not progress previous meiotic arrest, unable to execute germinal vesicle breakdown (GVBD) or extrude the primary polar physique. Among the many minority that proceeded, chromosomal misalignments and disorganized spindle assemblies have been prevalent, typically culminating in misguided ploidy upkeep. Furthermore, oocytes from pure matings exhibited dramatically lowered fertilization effectivity, with fertilized embryos arresting predominantly on the zygote stage, not often advancing past the two-cell stage.
To attach these mobile impediments with underlying molecular dysfunction, the crew assessed chromatin group and accessibility. They reported substantial enlargement of nucleolus-like our bodies (NLBs) in DHX36-deficient oocytes, accompanied by diminished heterochromatin condensation and world discount in key histone methylation marks—H3K4me3, H3K9me3, and H3K27me3. Concomitantly, the heterochromatin-associated protein HP1α adopted a diffuse nuclear distribution, indicative of disrupted heterochromatin upkeep. ATAC-seq information additional demonstrated decreased chromatin accessibility at transcription begin websites and ribosomal DNA (rDNA) areas, each plentiful in G4 motifs, implicating DHX36-mediated G4 decision in chromatin openness.
Exploring transcriptomic landscapes by way of Good-seq2 RNA sequencing of oocytes throughout developmental timepoints, the researchers recognized signatures of transcriptional insufficiency and faulty maternal transcript clearance in DHX36-CKO samples. Gene ontology enrichment emphasised dysregulation in DNA transcription issue exercise, ribonucleoprotein meeting, and rRNA biogenesis pathways. Notably, precursors of rRNA exhibited aberrant accumulation in totally grown oocytes (FGO) in comparison with rising oocytes (GO), paralleled by markedly lowered translational exercise, suggesting an intricate hyperlink between DHX36 operate, rRNA metabolism, and world protein synthesis.
On the coronary heart of the mechanistic investigation was the demonstration of G4 construction accumulation throughout the nucleolus and nucleoplasm of DHX36-deficient oocytes utilizing the G4-specific fluorescent probe CYTO and BG4 antibody. In vitro assays validated the formation of secure G4 constructions inside rDNA coding sequences similar to pre-rRNA transcripts, and biochemical interactions confirmed direct binding between DHX36 and pre-rRNA by way of its RSM area. These findings led to the compelling speculation that DHX36 resolves G4 formations on rDNA, thereby facilitating correct transcription and processing of pre-rRNA.
To look at the implications of stabilized G4 constructions on rRNA processing, cells have been handled with the G4 stabilizing ligand pyridostatin (PDS) in vitro. This remedy rendered pre-rRNA transcripts extra proof against RNase digestion, underscoring how G4 stabilization impedes regular RNA turnover. Spatial colocalization research of DHX36, pre-rRNA, and G4 constructions in early embryonic levels strengthened the practical interaction amongst these elements. Additional, RNA immunoprecipitation coupled with qPCR substantiated direct DHX36-pre-rRNA interactions.
Critically, reintroduction of DHX36 into CKO oocytes reversed pre-rRNA accumulation, confirming its pivotal function in sustaining pre-rRNA homeostasis. The research culminated within the paradigm that within the absence of DHX36, extreme G4 constructions accumulate, obstructing pre-rRNA cleavage, ribosome meeting, and translational competency—processes very important for oocyte maturation and embryonic development.
This seminal work outlines a complicated mechanism whereby DHX36 governs chromatin transforming and ribosome biogenesis by G4 decision, intricately linking nucleic acid secondary construction dynamics to developmental competence in mammalian oocytes. The findings present a molecular framework to grasp infertility related to disruptions in chromatin structure and rRNA metabolism and recommend DHX36 as a possible therapeutic goal for addressing reproductive dysfunctions linked to helicase deficiencies.
The research’s integration of superior genomic, biochemical, and imaging methodologies supplies a complete perspective on the epigenetic and transcriptional penalties of DHX36 loss. By inserting G4 constructions on the nexus of chromatin accessibility and rRNA processing, it prompts future analysis into G4-targeted interventions for correcting developmental defects. Furthermore, the revelations about nucleolar biology and ribosomal meeting underscore the broader significance of helicase-mediated nucleic acid transforming in mobile differentiation and adolescence formation.
In conclusion, the meticulous dissection of DHX36’s function in mouse oocytes underscores the helicase’s indispensable operate in facilitating correct chromatin conformation, empowering transcriptional resilience, and safeguarding the constancy of ribosome biogenesis. This research builds a compelling narrative the place DHX36 emerges as a guardian of genomic and epigenomic integrity, orchestrating the molecular choreography important for oocyte viability and embryonic success.
Topic of Analysis: The function of the helicase DHX36 in chromatin transforming and pre-rRNA processing throughout mouse oocyte growth and early embryogenesis
Article Title: DHX36 deficiency disrupts chromatin construction and impairs pre-rRNA processing in mouse oocytes
Information Publication Date: Not specified
Net References: http://dx.doi.org/10.1016/j.scib.2025.02.017
Picture Credit: ©Science China Press
Key phrases: DHX36, G-quadruplex, chromatin accessibility, nucleolus, pre-rRNA processing, oocyte growth, meiosis, ribosome biogenesis, infertility, mouse mannequin, ATAC-seq, RNA-seq
Tags: chromatin structure and gene expressionDHX36 helicase function in feminine fertilityearly embryogenesis and chromatin dysregulationG-quadruplex constructions in oocyte developmentG4 constructions and nucleic acid metabolismimplications of G4 constructions in copy.molecular biology of oocyte maturationmouse oocyte development mechanismsreproductive science and infertilityribosomal RNA processing in embryosspecialized helicases in developmental biologytranscriptional regulation by helicases




