
In a groundbreaking development in ovarian most cancers therapeutics, researchers have unveiled a pioneering gene immunotherapy method leveraging adeno-associated virus (AAV) vectors mixed with CRISPR/Cas9 genome enhancing expertise to immediately goal and disrupt PD-L1 expression inside tumor cells. This progressive technique addresses the persistent challenges confronted by standard antibody therapies aimed toward immune checkpoint molecules, which have traditionally exhibited restricted response charges in combating ovarian malignancies.
Ovarian most cancers poses vital therapeutic hurdles as a consequence of its immunosuppressive tumor microenvironment, which frequently undermines the efficacy of immune checkpoint inhibitors. The protein programmed demise ligand 1 (PD-L1), regularly overexpressed on ovarian tumor cells, performs a pivotal function in facilitating immune escape by interacting with PD-1 receptors on T cells, resulting in their purposeful exhaustion. By exactly ablating PD-L1 on the genomic stage, the brand new remedy strives to reinvigorate anti-tumor immune responses, providing a transformative route past standard antibody blockade.
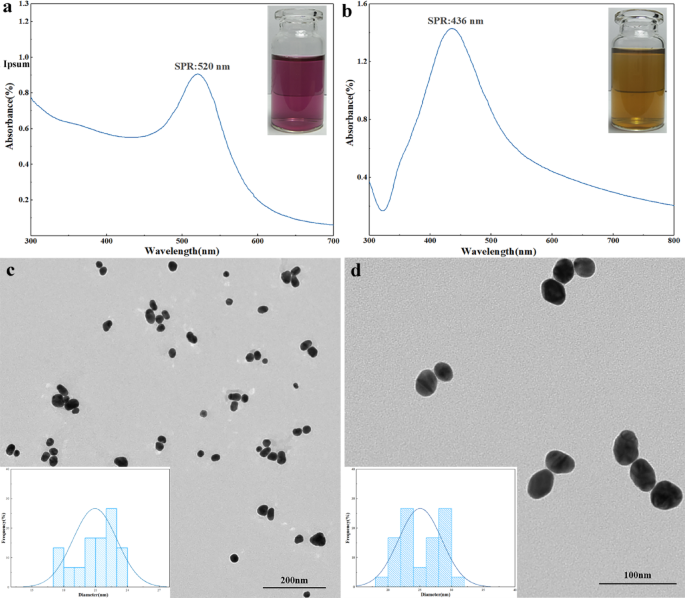
The analysis staff engineered an AAV vector system able to delivering CRISPR/Cas9 elements particularly designed to focus on and knockout the PD-L1 gene in ovarian most cancers cells. The selection of AAV as a supply platform is strategic, given its well-characterized security profile, low immunogenicity, and environment friendly transduction capabilities in vivo. Importantly, this viral vector-mediated gene enhancing method circumvents the transient nature and systemic toxicity limitations generally related to antibody administration.
In vitro experimentation concerned producing PD-L1-targeted AAV particles and subsequently transducing them into the murine ovarian most cancers cell line ID8. Put up-treatment analyses revealed a marked and statistically vital suppression of PD-L1 expression on the mobile stage when put next towards management teams handled with non-targeting AAV vectors. This clear demonstration of efficient gene knockout established a foundational proof-of-concept for the therapeutic potential of the technique.
Shifting past cell tradition, the examine employed a peritoneal dissemination mannequin of ovarian most cancers, which carefully mimics the medical presentation of metastatic illness inside the peritoneal cavity. Mice receiving intraperitoneal injections of PD-L1-targeting AAV particles exhibited considerably extended survival relative to control-treated counterparts. This survival profit underscored the purposeful influence of PD-L1 gene disruption on tumor development and host immunity in a residing organism.
Crucially, immunohistochemical analyses make clear the immunological dynamics inside the tumor microenvironment following gene enhancing intervention. A pronounced improve in intratumoral CD4+ helper T cells and CD8+ cytotoxic T lymphocytes was noticed in handled mice, a sample per reactivation of anti-tumor immune responses. Conversely, ranges of Foxp3+ regulatory T cells, which usually suppress immune exercise, had been notably decreased, suggesting an immunological shift favoring tumor eradication.
The protection profile of this gene-editing method was rigorously assessed by histological examination of main regular organs together with lungs, spleen, liver, and kidneys. Absence of extreme hostile results or off-target tissue injury was confirmed, bolstering confidence within the translational viability of AAV-CRISPR-based ovarian most cancers immunotherapy. The focused nature of the remedy minimizes collateral injury and systemic toxicity, one of many power limitations inherent to traditional chemotherapy and antibody therapies.
This examine highlights the immense promise of coupling genome enhancing applied sciences with viral supply programs to beat intrinsic immunotherapeutic resistance in ovarian most cancers. By leveraging the precision of CRISPR/Cas9 to completely disable immune checkpoint molecules like PD-L1, researchers can successfully dismantle the tumor’s immune suppressive defend and impress endogenous immune cells to assault malignant cells extra robustly.
Furthermore, the utilization of AAV vectors presents scalable and clinically related supply that may very well be tailored for human sufferers. Provided that AAVs have been extensively studied in gene remedy trials, their repurposing for most cancers immunotherapy represents a logical extension of current vector applied sciences. The relative stability and long-term expression facilitated by AAVs align effectively with the sustained anti-tumor immune activation required for sturdy remission.
A further benefit of this method is the potential to cut back the necessity for repetitive antibody dosing, thereby diminishing remedy burden, infusion-related hostile occasions, and financial prices related to present immunotherapeutic regimens. By delivering a one-time gene-editing remedy that exerts persistent suppression of PD-L1 expression, affected person outcomes and high quality of life may see substantive enchancment.
The rise in effector T cell infiltration mixed with decreased immunosuppressive Treg populations additional signifies a reprogramming of the tumor milieu in direction of heightened immunogenicity. This shift could sensitize tumors to extra therapeutic modalities, together with vaccines or small molecule immune modulators, creating avenues for mixture therapies that maximize anti-cancer efficacy.
Trying ahead, will probably be important to guage the long-term genomic stability, off-target results, and immune paradoxes related to CRISPR/Cas9-based enhancing in medical settings. Nonetheless, the present outcomes present a compelling basis for transitioning this technique into translational and medical analysis pipelines aimed toward tackling refractory ovarian most cancers circumstances.
Within the broader context of most cancers immunotherapy, this examine exemplifies a paradigm shift the place focused genetic disruption of immune inhibitory pathways could be exactly orchestrated in vivo, circumventing many pitfalls attribute of protein-based inhibitors. Such technological convergence opens a frontier for tailor-made, patient-specific therapeutic improvements rooted in molecular drugs.
In the end, the combination of AAV supply programs with CRISPR/Cas9-mediated genome enhancing may herald a brand new period in oncological therapies, the place anti-tumor immunity is enhanced by bespoke genetic interventions somewhat than systemic pharmacologic blockade alone. This method aligns effectively with the continuing evolution of personalised drugs and the hunt to realize lasting cures in difficult-to-treat malignancies like ovarian most cancers.
As medical trials and additional preclinical research advance, the scientific and medical communities will keenly observe the development of gene-based immune checkpoint modulation methods. The potential for reworking ovarian most cancers from a deadly illness right into a manageable situation is nearer than ever, pushed by improvements that manipulate tumor-immune interactions at their genomic roots.
The findings additionally increase intriguing questions on increasing related genome-editing immunotherapies to different strong tumors with excessive PD-L1 expression and inherent resistance to immune checkpoint inhibition. This platform expertise may revolutionize therapeutic landscapes throughout a number of most cancers varieties, shifting the paradigm from inhibition to eradication by engineered gene disruptions.
In abstract, the AAV-CRISPR/Cas9-mediated knockout of PD-L1 represents a formidable leap ahead in ovarian most cancers remedy methods. By elevating the immune system’s capability to detect and assault tumors at a molecular stage, this progressive gene immunotherapy holds super potential to boost survival outcomes and redefine the requirements of look after sufferers worldwide.
Topic of Analysis: Ovarian most cancers gene immunotherapy concentrating on PD-L1 utilizing AAV-CRISPR/Cas9 genome enhancing
Article Title: Adeno-associated virus-clustered often interspaced brief palindromic repeats/cas9‑mediated ovarian most cancers remedy concentrating on PD-L1
Article References:
Yahata, T., Toujima, S., Sasaki, I. et al. Adeno-associated virus-clustered often interspaced brief palindromic repeats/cas9‑mediated ovarian most cancers remedy concentrating on PD-L1. BMC Most cancers 25, 749 (2025). https://doi.org/10.1186/s12885-025-14093-0
Picture Credit: Scienmag.com
DOI: https://doi.org/10.1186/s12885-025-14093-0
Tags: AAV gene remedy for ovarian canceradeno-associated virus supply systemcancer immunotherapy advancementsCRISPR/Cas9 PD-L1 targetingenhancing anti-tumor immune responsegene enhancing in most cancers treatmentimmune checkpoint inhibition in ovarian cancerinnovative therapies for resistant cancersnovel immunotherapy for ovarian malignanciesovercoming immunosuppressive tumor microenvironmentPD-L1 knockout strategyprecision drugs in oncology