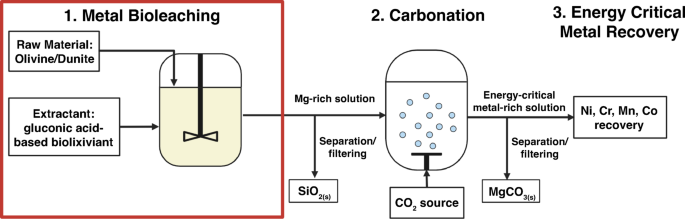
On this work, we carry out a examine of G. oxydans bioleaching functionality on dunite, an ultramafic rock composed of higher than 90% olivine (see composition in Determine S2, and Tables S1 and S2). For accelerated weathering applied sciences, olivine-rich dunite is the popular feedstock attributable to olivine having the very best CO2 sequestration potential by mass, glorious carbonation kinetics, and excessive abundance58,59.
This examine benchmarks the bioleaching efficacy of glucose-based and lignocellulosic sugar-based biolixiviants produced by G. oxydans on dunite, evaluating circumstances of whole-cell and filtered (cell-free) biolixiviants, various pulp densities, cardio and anaerobic circumstances, and short-term and long-term leaching. This examine additionally investigates how a high-performing mutant pressure of G. oxydans might enhance leaching in ultramafic minerals, serving as a preliminary examine of how genetic engineering might additional enhance bioleaching for carbon mineralization. We exhibit that the effectiveness benefit of the G. oxydans biolixiviant over gluconic acid alone grows with rising pulp density. Lastly, we exhibit that the variety of carbon atoms that must be equipped by sugar feedstocks will be diminished to match the variety of carbon atoms probably sequestered as a mineral.
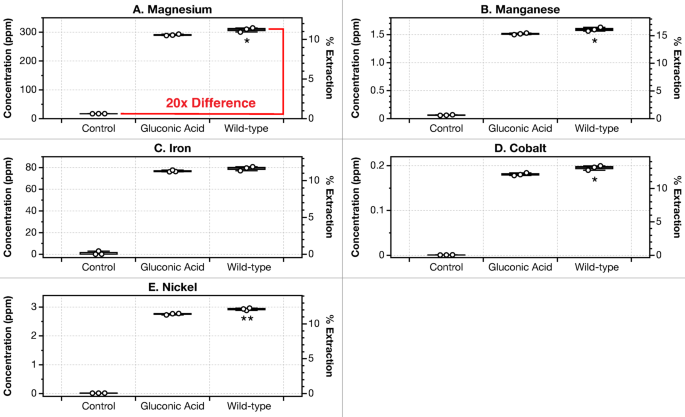
G. oxydans biolixiviants speed up magnesium and energy-critical metallic leaching by 20× over deionized water after 24 h
Biolixiviants from wild-type Gluconobacter oxydans successfully leached magnesium from ultramafic rocks (Fig. 2A). The effectivity of extraction measures how a lot of the out there metallic is leached from the mineral, and is calculated from the ratio of metallic concentrations within the leachate and the unique focus within the supply multiplied by the pulp density (ratio of stable mass to liquid quantity [g/L]),
$$:{eta:}_{textual content{e}textual content{x}textual content{t}textual content{r}textual content{a}textual content{c}textual content{t}textual content{i}textual content{o}textual content{n}}=frac{{C}_{textual content{M}textual content{g},textual content{l}textual content{e}textual content{a}textual content{c}textual content{h}textual content{a}textual content{t}textual content{e}}}{{C}_{textual content{M}textual content{g},textual content{d}textual content{u}textual content{n}textual content{i}textual content{t}textual content{e}}{rho:}_{textual content{p}textual content{u}textual content{l}textual content{p}}}$$
Deionized water is used as a management for these experiments because it estimates the enhancements made by bio-accelerated leaching to leaching from environmental water sources, comparable to rainwater. In conventional accelerated carbon mineralization with ultramafic rocks, crushed rocks are dispersed over land, the place water from the setting is the solvent for magnesium8,29,30. Evaluating the concentrations of magnesium between leaching with biolixiviants and water gives a tough estimate for the order of magnitude through which carbon mineralization is accelerated by G. oxydans.
After 24 h of leaching with dunite at 1% pulp density, magnesium was leached with an extraction effectivity of 11% (300 ppm), akin to a rise of 20× when in comparison with the deionized water management (Fig. 2A). The pH of wild-type biolixiviants was 2.39 ± 0.02 throughout all experiments utilizing wild-type biolixiviants. At the beginning of every leaching experiment, the preliminary concentrations of all metals in biolixiviants and controls are under detection limits. Determine S3 exhibits how the metallic concentrations in controls and G. oxydans leachates diverge inside the first few hours of leaching, beginning with preliminary metallic concentrations of zero.
Leaching dunite at 1% pulp density with wild-type (WT) G. oxydans biolixiviant improves leaching of magnesium by 20-fold when in comparison with leaching with deionized water (management). Nevertheless, the G. oxydans biolixiviant is just 6% higher than simply gluconic acid on the identical pH. Bioleaching of dunite at 1% pulp density shaking at 22 ˚C for twenty-four h was carried out with wild-type G. oxydans biolixiviant, gluconic acid diluted to the identical pH with deionized water, and a deionized water management. Panels (A) to (E) present leaching concentrations and extraction efficiencies of Mg, Mn, Fe, Co, and Ni. Wild-type G. oxydans biolixiviants barely improved metallic leaching in comparison with gluconic acid throughout all metals. Stars denote vital distinction between gluconic acid leaching and wild-type biolixiviant leaching by a Welch’s two-sample t-test, the place, p p p
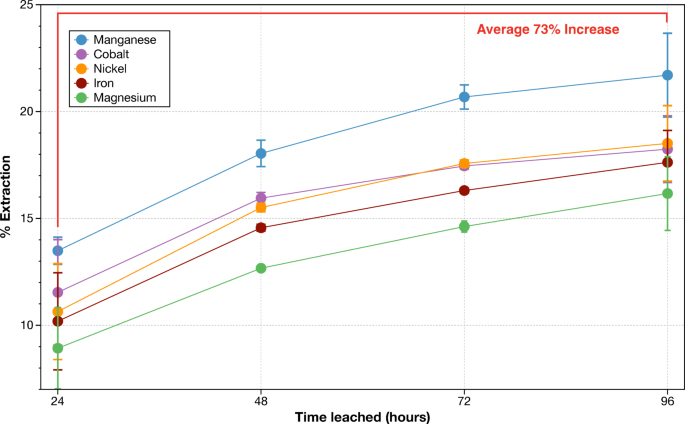
Bioleaching of metals by G. oxydans will increase by 73% from 24 to 96 h
Probably the most effectively extracted metallic at 24 h was Mn (Fig. 2B), extracting over 15% of Mn from dunite (akin to a leachate focus of 1.6 ppm). Fe, Co, and Ni had been additionally leached extremely, with efficiencies between 11% and 15% (Figs. 2C, D, and E, respectively). Al and Cr had been additionally leached, however a lot much less effectively (Determine S4). Cr was the least effectively extracted metallic, with extraction of not more than 0.3% underneath any situation (Determine S4 proper column). We speculate that chromium is considerably much less leached because the chromite inclusions inside dunite are comparatively extra secure than olivine and immune to acid dissolution60.
When leaching for longer than 24 h, bioleaching effectivity continues to extend (Fig. 3). In a 96-hour bioleaching evaluation of dunite at 1% pulp density, bioleaching elevated between 24 and 96 h by a median of 73% for Mg, Mn, Fe, Co, and Ni (Fig. 3). After 96 h, the leaching of magnesium in biolixiviant is 42 occasions that of water (Fig. 3). This exhibits that biolixiviants from G. oxydans enhance the general leaching of metals at a fee sooner than water.
Rising leaching time to 96 h will increase extraction effectivity by a median of 73% between 24 and 96 h for Mg, Mn, Fe, Co, and Ni. Biolixiviants from wild-type G. oxydans leached dunite at 1% pulp density for 96 h, shaking at 22 °C. Mg, Mn, Fe, Co, and Ni concentrations had been measured from leachates each 24 h for 96 h. Error bars point out commonplace deviation of measurements from duplicate measurements.
The G. oxydans biolixiviant is just 6% higher than gluconic acid at 1% pulp density
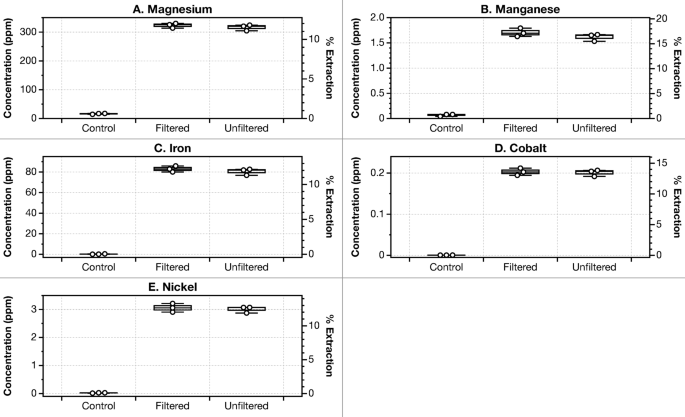
A secondary management was carried out utilizing solely gluconic acid diluted with deionized water to the identical pH because the acid biolixiviants produced by G. oxydans. Reed et al.42 and Antonick et al.49 demonstrated that biolixiviants produced by G. oxydans had been noticeably simpler at bioleaching than gluconic acid alone. Nevertheless, current anecdotal proof suggests this isn’t the case underneath all circumstances. In our palms, when bioleaching ultramafic supplies at low pulp density (1%), G. oxydans-produced biolixiviants solely barely outperform gluconic acid in leaching most metals (Fig. 2). Wild-type G. oxydans biolixiviants leached solely 6% extra magnesium in comparison with pure gluconic acid that was diluted with ultrapure water to the identical pH after 24 h of leaching. Moreover, not less than with low pulp density leaching, filtering cells from the biolixiviant didn’t considerably have an effect on bioleaching, not less than underneath the circumstances of those assessments (Fig. 4). This was executed to evaluate if there was a major residual impact of microbial exercise on leaching after biolixiviant manufacturing. Moreover, stoppering flasks throughout leaching to stop fuel change didn’t considerably have an effect on bioleaching (Determine S5). Later within the article, we ask if there are circumstances underneath which the G. oxydans biolixiviant has a major efficiency benefit for bioleaching ultramafic supplies.
Filtering cells from the G. oxydans biolixiviant doesn’t notably scale back the bioleaching of metals from dunite after 24-hour leaching. We in contrast Bioleaching of 1% dunite by sugar water (col. 1), with entire cell lixiviant (col. 2), and with a biolixiviant that was clarified by filtration with a 0.22 μm syringe filter (col. 3). Filtered and unfiltered biolixiviants had been at similar pH, produced from duplicate colonies, and had been added to ultramafic minerals in similar circumstances. Filtering biolixiviants earlier than bioleaching resulted in comparable metallic concentrations when in comparison with whole-cell biolixiviants when leaching dunite. A Welch’s two-sample t-test exhibits that no vital distinction was detected between the unfiltered and filtered teams, with p-values starting from 0.2 to 0.9. This means that based mostly on a 24-hour leaching examine, the presence of G. oxydans doesn’t considerably enhance or diminish the impact of the biolixiviant on leaching briefly time period leaching.
Cellulosic hydrolysate can be utilized in its place sugar feedstock for bioleaching
Techno-economic evaluation exhibits that glucose feedstock for biolixiviant manufacturing is the most important value driver in REE biomining with G. oxydans, accounting for nearly half of the entire value61. Moreover, the sheer scale that carbon sequestration must function at, signifies that huge quantities of feedstock will likely be required, probably putting a major demand on the world’s biomass provide62.
Lignocellulose, present in agricultural waste, is usually a renewable carbon supply for microbial progress and manufacturing as soon as monomeric sugars are launched via acid pretreatment and enzymatic hydrolysis, making a glucose-rich cellulosic hydrolysate63,64. Lignocellulosic sugars are being more and more investigated as a possible different sugar supply for a number of purposes, together with biofuels/bioethanol manufacturing and substrates for mobile manufacturing. Using lignocellulose-derived sugars might function a excessive glucose substitute feedstock that might enormously enhance the cost-efficiency of bioleaching for carbon seize applied sciences65,66. G. oxydans has been proven to have the ability to convert the glucose and xylose sugars in cellulosic hydrolysate into gluconic acid and xylonic acid at extraordinarily excessive efficiencies67,68,69. Changing pure glucose with cellulosic hydrolysate might additional enhance the financial and environmental viability of bio-accelerated weathering.
G. oxydans has been proven to effectively produce gluconic acid and xylonic acid from lignocellulosic hydrolysate feedstocks67,68,69, making industrial-scale, bio-accelerated carbon mineralization much more economically possible. We investigated if biolixiviants produced from cellulosic hydrolysate, somewhat than pure glucose, might be efficient at leaching dunite. The cellulosic hydrolysate (CH) used on this examine is a glucose-rich resolution produced from the enzymatic hydrolysis of concentrated corn stover (Desk S3).
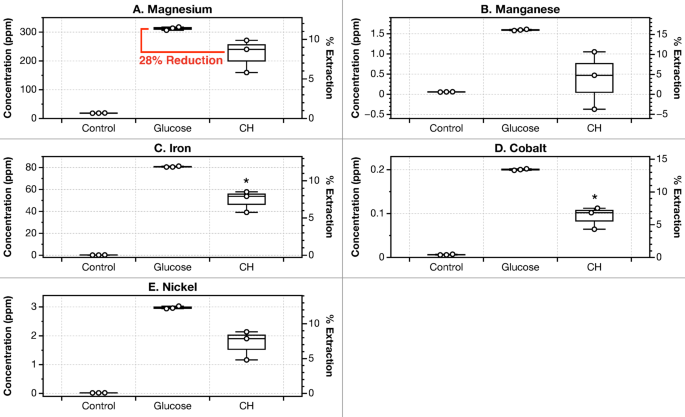
Biolixiviants produced from cellulosic hydrolysate had been on common 28% much less efficient in leaching magnesium in comparison with glucose-based biolixiviants (Fig. 5). Leaching of Mn, Fe, Co, and Ni was additionally diminished by a median of 52% in comparison with glucose-based biolixiviants (Fig. 5). Dunite was added at 1% pulp density, and a leaching comparability between CH-based and glucose-based lixiviants was carried out in parallel and in similar circumstances. The cellulosic hydrolysate added was diluted with deionized water in order that the entire sugar concentrations had been equal to the quantity of glucose added for glucose-based biolixiviants.
Use of cellulosic hydrolysate as a biolixiviant feedstock doesn’t significantly scale back bioleaching effectivity. Panels (A) to (E) present comparability of bioleaching of Mg, Mn, Fe, Co, and Ni by Gluconobacter oxydans when utilizing glucose and cellulosic hydrolysate (CH) as as biolixiviant feedstock. CH-based biolixiviant leaches magnesium on common at 72% the efficacy of glucose-based biolixiviant. Leaching was carried out with 1% dunite shaking at 22 °C for twenty-four h. Stars denote vital distinction in contrast with glucose-based biolixiviants by a Welch’s two-sample t-test, the place, p p p
Regardless of a 28% common discount in Mg leaching, we had been unable to detect a statistically significant distinction between glucose- and CH-based leaching of Mg utilizing a Welch’s two-sample t-test. This was as a result of small pattern measurement and variability in CH-based biolixiviant leachate measurements. Information collected for CH-based biolixiviants resulted in higher variability than information with glucose-based biolixiviants. ICP-MS evaluation of cellulosic hydrolysate samples confirmed that the CH used on this examine comprises hint concentrations of metals, presumably on account of the manufacturing course of (Desk S4). When calculating the quantities of metallic leached from CH-based biolixiviants, the concentrations of metallic in a cellulosic hydrolysate management examine had been subtracted from the entire detected to eradicate metallic concentrations that weren’t extracted from dunite. In low pulp density research, the concentrations of metals within the cellulosic hydrolysate feedstock interfered with metals leached, leading to larger total error.
Cellulosic hydrolysate produced from corn stover comprises a number of toxins comparable to natural acids, furan derivatives, and phenolic compounds that would inhibit the expansion of G. oxydans and its manufacturing of biolixiviant70. Cellulosic hydrolysate additionally considerably will increase viscosity, leading to decrease oxygen switch charges that restrict the fermentation of glucose to gluconic acid68. Regardless of this, leaching outcomes present that G. oxydans can produce a biolixiviant from cellulosic hydrolysate able to leaching metals at concentrations considerably higher than controls; nonetheless, additional evaluation and experimentation are wanted to point out if CH is usually a viable substitute to glucose with out a vital discount in efficiency.
Though cellulosic hydrolysate comprises gentle antibiotic properties attributable to its acidity and sure poisonous compounds, this might be advantageous for the commercial scale use of it as a feedstock for biolixiviant manufacturing. G. oxydans has excessive acid resistance and is able to rising and producing biolixiviant with cellulosic hydrolysate, whereas many different microbes have low tolerance mechanisms for it. This might permit for cellulosic hydrolysate for use as an ultra-low-cost feedstock whereas additionally stopping the expansion of competing microbes. The poisonous elements of cellulosic hydrolysate is also a genetic engineering goal for G. oxydans, the place mutants which have higher resistance mechanisms will be chosen for to enhance biolixiviant manufacturing.
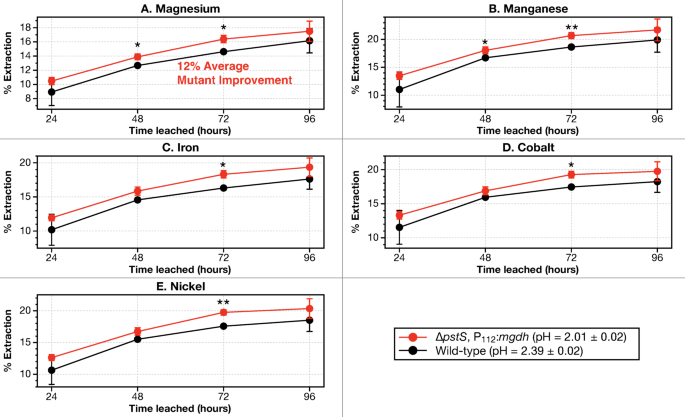
G. oxydans mutant pressure ΔpstS, P112:mgdh improved magnesium leaching by 12% over wild-type
A preliminary examine utilizing the mutant pressure G. oxydans ΔpstS, P112:mgdh43 exhibits that underneath similar progress and leaching circumstances, mutant strains of G. oxydans will be simpler than wild-type in leaching magnesium and energy-critical metals from dunite.
A bioleaching evaluation over 96 h utilizing dunite at 1% pulp density was carried out with biolixiviant from wild-type and mutant G. oxydans. Bioleachate samples had been taken and analyzed each 24 h as much as 96 h utilizing ICP-MS. The pressure used is G. oxydans ΔpstS, P112:mgdh, which was engineered to enhance acid manufacturing for metallic leaching43. It comprises a clear deletion of the pstS gene which is concerned in phosphate signaling and transport44, and an up-regulation with promoter P112 of the mgdh gene (Membrane-bound Glucose DeHydrogenase) which is crucial for changing glucose into gluconic acid71. The ΔpstS, P112:mgdh mutant was beforehand proven to lower the pH of biolixiviants in comparison with wild-type, and it will increase the general leaching of REE43. The biolixiviant produced from the ΔpstS, P112:mgdh mutant had a pH of two.01 ± 0.02 throughout all replicates, whereas the pH of wild-type biolixiviants had been 2.39 ± 0.02.
The evaluation exhibits that ΔpstS, P112:mgdh mutant biolixiviant will increase magnesium leaching by a median of 12% over all samples taken between 24 and 96 h of leaching. Mn, Fe, Co, and Ni had a median enchancment in leaching of 12% utilizing mutant biolixiviant in comparison with wild-type (Fig. 6).
Bioleaching effectivity of magnesium elevated by a median of 12% when leaching with a excessive performing G. oxydans mutant. The G. oxydans mutant comprises a deletion of pstS phosphate signaling and transport gene, alongside up-regulation of the mgdh gene that codes for the Membrane-bound Glucose DeHydrogenase (MGDH) with the P112 promoter (G. oxydans ΔpstS, P112:mdgh). Leaching was executed with dunite at 1% pulp density shaking at 22 °C over 96 h. Stars and dots denote a statistically vital distinction between wild-type G. oxydans and G. oxydans ΔpstS, P112:mdgh mutant by a Welch’s two-sample t-test, the place, p p p p
The complete genome of G. oxydans is but to be fully characterised, and several other different genes might exist which can be crucial in controlling metallic leaching from ultramafic rocks. Future work to characterize the genome of G. oxydans when it comes to its capabilities associated to ultramafic leaching is important to engineer higher mutant strains that may enhance the efficiency of bio-accelerated weathering. The ΔpstS, P112:mgdh mutant is engineered to maximise gluconic acid manufacturing; nonetheless, research have proven that for uncommon earths leaching, G. oxydans mutants will be screened to establish genes that particularly have an effect on uncommon earth extraction50. Our analysis group has created a quality-controlled (QC) entire genome knockout assortment for G. oxydans B58, containing a group of two,733 transposon disruption mutants, every with a special nonessential gene disrupted. This QC assortment permits a high-throughput display screen of the G. oxydans genome, deciding on for vital modifications to dunite leaching efficacy. This may present us with a roadmap for genetically engineering G. oxydans for higher ultramafic bio-accelerated weathering functionality.
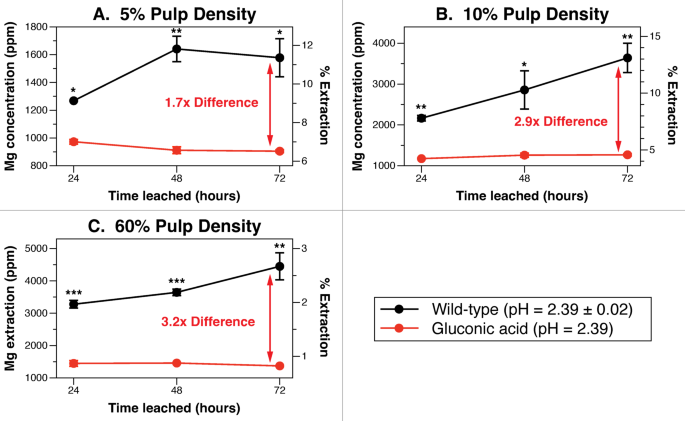
G. oxydans biolixiviants are 3.2× simpler than gluconic acid at 60% pulp density
At larger pulp densities and longer leaching occasions, Gluconobacter oxydans biolixiviants considerably outperformed gluconic acid on the identical pH. We in contrast Gluconobacter oxydans biolixiviants and gluconic acid metallic leaching with 5%, 10%, and 60% dunite pulp density, the place leachate metallic concentrations had been measured each 24 h for 72 h. The gluconic acid was diluted with ultrapure deionized water to match the pH of biolixiviants, and leaching was carried out in similar circumstances with the identical mineral substrates (DUN-2). After 72 h of leaching, wild-type biolixiviants leached 1.74 occasions extra magnesium than gluconic acid from 5% dunite, 2.87 occasions extra from 10% dunite, and three.25 occasions extra from 60% dunite (Fig. 7). At larger pulp densities, Gluconobacter oxydans biolixiviants exhibit considerably larger leaching effectivity than gluconic acid. This evaluation means that metallic leaching from dunite just isn’t solely managed by acidolysis, however may include different biologically-synthesized elements that amplify extraction50.
At 60% pulp density, the wild-type G. oxydans biolixiviant was 3.2× simpler at bioleaching dunite than gluconic acid diluted to the identical pH with pure water. A comparative bioleaching examine was carried out evaluating the leaching efficacy of wild-type G. oxydans biolixiviants and simply gluconic acid on the identical pH. Magnesium concentrations in leachate had been measured after 24, 48, and 72 h of leaching with 5% (A), 10% (B), and 60% (C) dunite (DUN-2). At larger dunite pulp densities, the advance of Gluconobacter oxydans biolixiviants in comparison with gluconic acid was amplified. After 72 h, biolixiviants had been 74% higher than gluconic acid at leaching 5% dunite, 1.87 occasions higher at leaching 10% dunite, and a pair of.25 occasions higher at leaching 60% dunite. Stars denote vital distinction between gluconic acid leaching and wild-type biolixiviant leaching by a Welch’s two-sample t-test, the place, p p p
Magnesium leaching relative to glucose enter will be enormously improved by course of and genetic engineering
Glucose is the cost-limiting think about using G. oxydans biolixiviant as a weathering accelerant61. Moreover, the sheer scale at which carbon sequestration should function requires huge quantities of microbial feedstock, presumably sufficient to make a major demand on the world’s biomass provide62. Subsequently, it’s important to prioritize maximizing carbon dioxide sequestration whereas minimizing feedstock consumption to be able to mitigate the pressure on worldwide biomass sources.
We outline the carbon funding to carbon sequestration ratio, okseq, because the variety of carbon atoms within the feedstock (e.g., glucose) wanted to liberate one magnesium ion (and therefore sequester the only atom of carbon in a CO2 molecule11,62,
$$:{ok}_{textual content{s}textual content{e}textual content{q}}=frac{textual content{C}textual content{a}textual content{r}textual content{b}textual content{o}textual content{n}:textual content{i}textual content{n}textual content{p}textual content{u}textual content{t}:left(textual content{f}textual content{r}textual content{o}textual content{m}:textual content{g}textual content{l}textual content{u}textual content{c}textual content{o}textual content{s}textual content{e}:textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}proper)}{textual content{C}textual content{a}textual content{r}textual content{b}textual content{o}textual content{n}:textual content{t}textual content{h}textual content{a}textual content{t}:textual content{c}textual content{o}textual content{u}textual content{l}textual content{d}:textual content{b}textual content{e}:textual content{s}textual content{e}textual content{q}textual content{u}textual content{e}textual content{s}textual content{t}textual content{e}textual content{r}textual content{e}textual content{d}:textual content{b}textual content{y}:textual content{r}textual content{e}textual content{a}textual content{c}textual content{t}textual content{i}textual content{n}textual content{g}:textual content{w}textual content{i}textual content{t}textual content{h}{:textual content{M}textual content{g}}^{2+}}$$
Decrease okseq values point out that extra magnesium was leached given a sure glucose enter, whereas excessive okseq values point out comparatively decrease magnesium leaching for a similar quantity of feedstock consumed. A okseq worth of 6 for a glucose feedstock signifies that one glucose molecule is important to liberate one magnesium ion (which has the potential to sequester 1 CO2). A okseq worth of 1 signifies that a minimal of 1 feedstock carbon atom is required to finally sequester one CO2 molecule. The assertion that every liberated magnesium ion ends in one CO2 molecule sequestered assumes a 100% carbonation fee in a future downstream course of that converts magnesium in leachate into magnesite. okseq solely illustrates the decrease restrict of sugar enter essential to sequester CO2.
We will use okseq to estimate the decrease restrict value of carbon sequestration and the entire quantity of feedstock wanted to sequester a helpful quantity of CO2 (e.g., 20 gigatonnes of CO2 per 12 months). The mass of feedstock, Mfeedstock, wanted to sequester a given mass of CO2, (:{M}_{{CO}_{2}}),
$$:{M}_{textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}=left(frac{{textual content{M}textual content{W}}_{textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}}{{textual content{M}textual content{W}}_{{textual content{C}textual content{O}}_{2}}}proper)left(frac{{M}_{{textual content{C}textual content{O}}_{2}}{ok}_{textual content{s}textual content{e}textual content{q}}}{{n}_{textual content{C},textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}}proper)$$
the place, MWfeedstock is the molecular weight of the feedstock; (:{textual content{M}textual content{W}}_{{textual content{C}textual content{O}}_{2}}) is the molecular weight of CO2, and nC, feedstock is the common variety of carbon atoms in every molecule of the feedstock. Likewise, the fee to sequester a given mass of CO2,
$$:{P}_{{textual content{C}textual content{O}}_{2}}=left(frac{{textual content{M}textual content{W}}_{textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}}{{textual content{M}textual content{W}}_{{textual content{C}textual content{O}}_{2}}}proper){M}_{{textual content{C}textual content{O}}_{2}}left(frac{{ok}_{textual content{s}textual content{e}textual content{q}}}{{n}_{textual content{C},textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}}proper){p}_{textual content{f}textual content{e}textual content{e}textual content{d}textual content{s}textual content{t}textual content{o}textual content{c}textual content{ok}}$$
The place pfeedstock is the value per unit mass of the feedstock.
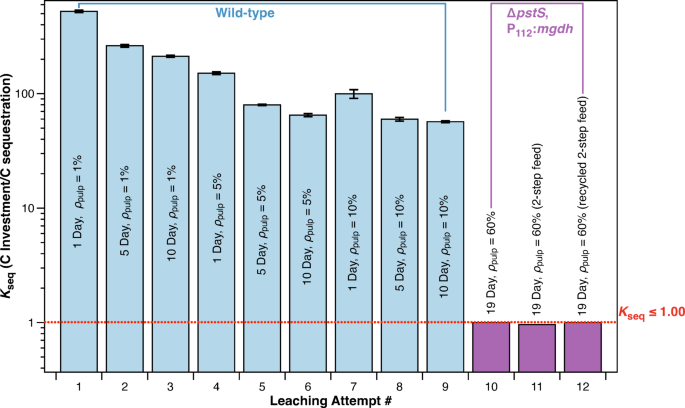
On this work, we succeeded in decreasing okseq from 525 to 1.0 (nearly 3 orders of magnitude) (Fig. 8). With 1% dunite and 24 h of leaching, the approximate okseq is 525 carbon invested/magnesium leached. One methodology to cut back okseq is to extend the time of leaching. After 96 h of leaching, the magnesium focus elevated by 81% when in comparison with 24 h leaching on the identical pulp density (Fig. 3). When rising the time of leaching to 10 days, the magnesium focus improved by a median of 147% (Fig. 8). Rising the pulp density of dunite additionally reduces okseq. When in comparison with 1% pulp density of dunite, rising to 10% pulp density resulted in a 5-fold improve in magnesium leaching after 24 h (Fig. 8). When combining 10% pulp density with 10 days of leaching, magnesium leaching elevated by 9.2 occasions that of 1% pulp density leached for 1 day (Fig. 8), leading to a okseq of 57, since glucose enter remained unchanged. For the bottom okseq values achieved, fine-particle dunite at a pulp density of 60% was leached for 19 days with three completely different strategies (Fig. 8 Leaching Makes an attempt #12–14). The primary methodology (Fig. 8 Leaching Try #12) was direct addition of 60% dunite, the second methodology (Leaching Try #13) was a 2-step dunite addition, the place 30% dunite was added on day 0 and one other 30% added on day 10, and the third methodology (Leaching Try #14) was a 2-step dunite addition the place the primary 30% dunite feed was faraway from the leachate earlier than the second 30% addition on day 10. All three leaching strategies resulted in a okseq equal to or lower than 1.0, with the bottom okseq being 0.96 on Leaching Try #13 in Fig. 8.
Course of and genetic enhancements scale back the quantity of feedstock carbon wanted to probably sequester one CO2 molecule from 525 to 1. Rising leaching time, pulp density, and decreasing common particle sizes had been capable of scale back okseq (glucose carbon funding vs. carbon sequestration ratio) by a number of orders of magnitude. Rising dunite pulp density from 1–10% had a bigger influence in decreasing okseq than rising leaching time from 1 to 10 days. Combining the ΔpstS, P112:mdgh double mutant with excessive pulp densities and lengthy leaching occasions resulted in a okseq worth of 1 in three experiments (Col. 10–12). Leaching makes an attempt #1–9 used DUN-1 and makes an attempt #10–12 used DUN-3 with a particle measurement of lower than 74 microns because the leaching substrate.
What implications do these outcomes have for CO2 sequestration? For okseq = 525, when utilizing glucose as a feedstock, the price of sequestering 1 tonne of CO2 is $358,000, a good distance above the US Division of Power’s (DOE) goal of $100 per tonne72, and effectively above current applied sciences31. However, if we might scale back okseq to 1 (as in Fig. 8) at industrial scale, the price of carbon sequestration (not less than the microbial feedstock a part of the fee) via bio-accelerated weathering begins to turn into corresponding to (nonetheless excessive) present applied sciences, at $682 per tonne31. If we might scale back okseq to 0.15 (a few 6-fold discount over our greatest try, a lot smaller than the discount we achieved in the midst of writing this text) then we might scale back the feedstock value for sequestering 1 tonne of CO2 to $100. This is able to not account for the prices of mining ultramafic materials, however nor does it account for the offset value of energy-critical metallic restoration. Nevertheless, utilizing this to sequester 20 gigatonnes of CO2 a 12 months5 would require 2 billion tonnes of glucose, over 10 occasions present world annual sugar manufacturing73.
Sugars derived from cellulosic biomass are probably rather more considerable than glucose that’s derived from cereal crops like corn, sugar cane, and sugar beet74. Whereas there isn’t a widespread manufacturing of cellulosic sugars at the moment that isn’t instantly diverted to cellulosic ethanol manufacturing, this might occur sooner or later. Moreover, it’s estimated that cellulosic sugar manufacturing might be as little as $100 per tonne75 (in distinction to round $1,000 per tonne for glucose). Though cellulosic sugars are considerable, they don’t seem to be limitless. Slade et al.76, made estimates of world bioenergy provide, and concluded that a number of tipping factors would happen as the quantity of biomass withdrawn from the biosphere for bioenergy (or another chemical manufacturing, comparable to carbon sequestration) elevated, forcing more and more unpalatable decisions on world society together with mass adoption of vegetarian diets, mass deforestation, inhabitants management, or combos thereof. Their evaluation means that the primary world agricultural tipping level (selecting one of many three unpalatable choices) would happen when biomass was withdrawn at a fee of round 7 gigatonnes per 12 months62. Assuming that cellulosic sugars are on common a 50:50 mixture of C5 and C6 sugars, then to cut back the cellulosic biomass demand to sequester 20 gigatonnes of CO2 to three.5 gigatonnes, okseq wants solely to be diminished to 0.25.
Though okseq serves as a helpful metric for displaying sugar funding when it comes to CO2 sequestration potential, it’s only a theoretical worth whose actual worth will rely on the conversion charges of downstream CO2 mineralization. Since glucose inputs are a significant value driver in bio-accelerated weathering61, okseq may also help estimate lower-limit prices of scaled operations. Future scale-up work will likely be essential to formulate extra correct estimates of value earlier than they are often in contrast with different direct CO2 mineralization applied sciences.
Potentialities for downstream carbon mineralization
By itself, G. oxydans bioleachate saturated with magnesium ions will likely be troublesome to transform into secure carbonate minerals. As a result of nature of acid leaching, the ultimate pH of biolixiviants post-leaching tended to be low (4–5) in our experiments, making carbon mineralization chemically unfavorable. Since pH has a major affect on the precipitation of carbonates as a result of decrease availability of carbonate ions in acidic circumstances77,78, pH changes are required to extend carbonate mineral yields downstream. Rising the focus of gaseous CO2 injected or sparged via the leachate will increase the entire dissolved inorganic carbon (DIC); nonetheless, this reduces the pH of the leachate, decreasing carbonate ion availability and lowering the saturation index of carbonate minerals2,77.
A attainable downstream pathway to regulate the pH of leachates to make carbon dioxide complexation chemically favorable is so as to add ammonia from organic or pure sources to synthesize magnesite, MgCO3, or nesquehonite, MgCO3·3H2O. Including ammonia to CO2-sparged magnesium chloride options at ambient temperatures was proven to fully convert free magnesium ions into nesquehonite in about ten minutes79. Moreover, ammonia will be recycled on this course of via restoration of ammonia utilizing activated carbon, which has been proven to get well ammonium chloride salts and separate them into ammonia and hydrochloric acid at close to ambient circumstances80. One other chance is to easily add sodium hydroxide that has been electrically synthesized from carbon-neutral pathways.
Microbes that speed up carbon mineralization, comparable to cyanobacteria, have been noticed to speed up mineralization in legacy alkaline mine tailings34,35. Coupling bio-accelerated weathering from Gluconobacter oxydans with biotic downstream processes might maximize carbonate precipitation in a low-resource and energy-efficient course of that might be genetically engineered additional.
Scale-Up and future steps
As a way to present that bio-accelerated weathering is a possible possibility for gigatonne-scale CO2 sequestration, future work will likely be required to point out {that a} scaled-up bioprocess is feasible with out a vital discount in effectivity in comparison with the bench-scale experiments proven right here.
Bench-scale lab outcomes not often have equal yields and efficiencies when scaled as much as pilot- or industrial-scale bioprocesses. Being an obligate aerobe, G. oxydans requires air change and constant mixing for optimum progress. Moreover, optimized mixing of the mineral substrate with biolixiviants, fee of glucose supplementation, fee of mineral addition, and optimized particle sizes for leaching are but to be completely investigated. To handle these scale-up challenges, all course of parameters will must be investigated in bioreactor experiments.
One other consideration is how cell viability modifications all through the leaching course of. G. oxydans has been proven to be resilient in excessive metallic concentrations11, in contrast to a number of different microbes. Nevertheless, having excessive pulp densities of dunite can be a progress problem for G. oxydans. Regardless of this, cell viability throughout leaching might not be a crucial parameter for the effectiveness of leaching by G. oxydans biolixiviants. For brief time period leaching, it’s proven that having cells current within the biolixiviant has an insignificant impact on leaching (Fig. 4). Since biolixiviants are produced in an unbiased step earlier than leaching begins (Determine S1), it’s not important to have dwell cells current throughout leaching. Nevertheless, attainable direct microbe-mineral interactions and continued acid manufacturing might impact long-term leaching and scaled processes.
Overcoming the deceleration of leaching over time can also be a future scale-up problem to deal with. The charges of leaching had been proven to decelerate over the 96 h they had been monitored (Fig. 3). As ultramafic minerals are dissolved by biolixiviants, passivation layers can kind from amorphous silica (Notice S1) or different mineral byproducts. Moreover, as the answer nears saturation of metallic cations, dissolution charges are anticipated to stagnate. Future investigations into overcoming the passivation layers and growing built-in mineralization applied sciences to permit for steady leaching will likely be vital for scale-up.
Using lignocellulosic-based sugars in its place sugar feedstock additionally poses a problem in scale-up. Though G. oxydans has been proven to successfully produce biolixiviants from cellulosic hydrolysate, the state of lignocellulosic sugar manufacturing is underdeveloped. Massive-scale manufacturing of sugars derived from second-generation biomass (lignocellulose) remains to be restricted, and developments in enhancing effectivity and yield are ongoing. Advances within the provide chain of lignocellulosic biomass and optimization in pretreatment and enzymatic hydrolysis will likely be vital within the close to future for the large-scale implementation of lignocellulosic-based sugars for bio-accelerated weathering66.