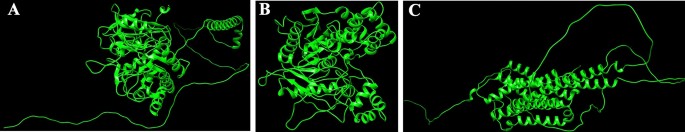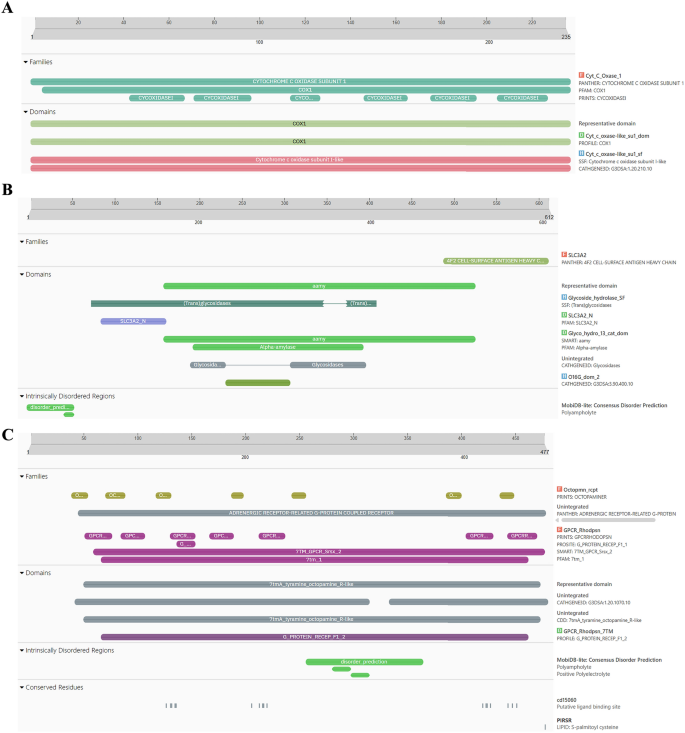
Homology modeling and high quality evaluation
Protein sequences for CEHs, AGI, and ORs had been modeled utilizing the SWISS-MODEL server. The 3D PDB fashions are represented in (Fig. 1A–C). For CEHs, the AlphaFold DB mannequin of A0A2H1VAI9_SPOFR, primarily based on the Spodoptera frugiperda (Fall Armyworm) sequence (Gene: SFRICE041916.2), was chosen because the template, exhibiting 89.87% sequence identification. The AGI mannequin was constructed utilizing the AlphaFold DB mannequin of A0A7E5VBV1_TRINI from Trichoplusia ni (Cabbage Looper) (Gene: LOC113492491), with a 70.10% sequence identification. For the ORs, the AlphaFold DB mannequin of OAR_HELVI from Heliothis virescens (Tobacco Budworm Moth) (Gene: OAR_HELVI) was used, exhibiting 96.22% sequence identification. The standard of the modeled protein buildings was validated utilizing the SAVES v6.0 Construction Validation Server. Ramachandran plot evaluation was carried out to evaluate the spine dihedral angles (phi and psi) of the residues throughout all three fashions. The evaluation revealed {that a} excessive proportion of residues in every mannequin occupy probably the most favored areas of the plot, with 87.4% for the AGI mannequin (Fig. 2A), 84.9% for the CEHs mannequin (Fig. 2B), and 85.5% for the ORs mannequin (Fig. 2C). Extra residues had been situated in allowed areas, with a minimal share present in generously allowed and disallowed areas (starting from 0.2 to 2.5%). Outliers comparable to Valine 563 within the AGI mannequin and Histidine 281 within the CEHs mannequin had been famous however characterize remoted circumstances that don’t considerably impression the general structural stability. Glycine and proline residues had been positioned as anticipated, additional supporting the structural integrity of the fashions. Total, the buildings are thought-about dependable, with solely minor refinements required for optimization. These fashions are well-suited for subsequent computational analyses, together with molecular dynamics simulations and docking experiments.
(A–C) The standard of the modeled protein structures- AGI, CEHs, and ORs was validated utilizing the SAVES v6.0 construction validation server by way of Ramachandran plot evaluation. (A) Ramachandran plot evaluation of SWISS modeled AGI PDB construction from SAVES v6.0 construction validation server, (B) Ramachandran plot evaluation of SWISS modeled CEHs PDB construction from SAVES v6.0 construction validation server, (C) Ramachandran plot evaluation of SWISS modeled ORs PDB construction from SAVES v6.0 construction validation server.
Purposeful area, power minimization, and energetic web site prediction
The proteins:COX1, AGI, ORs, CEHs, and CSPs had been analyzed utilizing InterPro to determine domains, homologous superfamilies, and useful motifs inside their sequences. The useful domains, homologous superfamily, and useful motifs inside a protein sequence evaluation of proteins- COX1, AGI, Ors, CEHs, and CSPs by way of InterPro had been represented in (Fig. 3A–E). The proteins exhibit exceptional structural and useful range, every taking part in a pivotal function in crucial organic processes. COX1 (Fig. 3A) is important for mobile respiration, particularly throughout the electron transport chain. The COX1 area on this protein drives power manufacturing by way of its involvement in proton pumping and electron switch. Its transmembrane and cytoplasmic areas assist environment friendly power transduction, facilitating electron stream and contributing to the technology of a proton gradient vital for ATP synthesis. This makes COX1 indispensable for mobile power metabolism, underscoring its integral function in oxidative phosphorylation43. AGI (Fig. 3B) is a key enzyme in carbohydrate metabolism, containing domains from the Glyco_hydro_13_cat_dom and SLC3A2 households. These domains allow the enzyme to hydrolyze starch and sugars by breaking glycosidic bonds, important for the conversion of complicated carbohydrates into glucose. Its transmembrane and cytoplasmic areas recommend its membrane-bound nature, positioning it to facilitate not solely carbohydrate processing but additionally the transport of sugars throughout mobile membranes, which is crucial for mobile power manufacturing and glucose homeostasis44. ORs protein (Fig. 3C) belongs to the GPCR (G-protein coupled receptor) superfamily, particularly throughout the ORs household. Characterised by the 7tmA, tyramine, and GPCR rhodopsin-like domains, this receptor mediates sign transduction throughout the cell membrane. The seven transmembrane helices facilitate the relay of exterior alerts to intracellular signaling pathways, regulating crucial physiological processes comparable to hormone response and neurotransmission. This makes the ORs central to modulating behaviors associated to emphasize, locomotion, and hormonal steadiness45. CEHs (Fig. 3D) characteristic a CO esterase area and a Carboxylesterase kind B area, which place it throughout the Alpha/Beta hydrolase fold superfamily. These domains point out its operate as a carboxylesterase enzyme, hydrolyzing ester bonds in xenobiotics and endogenous compounds. Its conserved serine energetic web site is significant for catalysis, making it a key participant in cleansing and metabolism. The presence of each cytoplasmic and non-cytoplasmic domains suggests the enzyme operates throughout numerous mobile environments, coordinating cleansing processes and metabolic regulation46. CSPs (Fig. 3E), a part of the Insect odorant-binding protein household and Csp2 superfamily, are concerned in detecting odorants and pheromones crucial for insect sensory biology. This protein performs a central function in chemical sign detection and transduction, which is significant for behaviors like foraging, mating, and navigation. Recognizing environmental alerts, allows organisms to reply to their environment, emphasizing its function in sensory adaptation and behavioral responses. Collectively, these proteins illustrate the delicate evolution of area structure and useful specificity, supporting important processes like hydrolytic exercise, power manufacturing, sensory sign transduction, and carbohydrate metabolism. Their structural complexity ensures the upkeep of mobile and physiological homeostasis throughout completely different organisms, highlighting the intricate mechanisms underlying life’s important features47.
(A–E) Purposeful domains, homologous superfamily, and useful motifs inside a protein sequence evaluation of proteins- COX1, AGI, ORs, CEHs, and CSPs by way of InterPro. (A) Purposeful area evaluation of COX1 sequence by way of InterPro, (B) Purposeful area evaluation of AGI sequence by way of InterPro, (C) Purposeful area evaluation of ORs sequence by way of InterPro, (D) Purposeful area evaluation of CEHs sequence by way of InterPro, (E) Purposeful area evaluation of CSPs sequence by way of InterPro.
Vitality minimization is a vital step in refining protein buildings for molecular dynamics simulations, because it helps to resolve steric clashes and optimize atomic positions, resulting in extra steady and correct fashions. Utilizing YASARA, vital power reductions had been noticed in a number of key proteins: COX1(−91,162.2 to −117,351 kJ/mol), AGI (−250,203.2 to −337,654.7 kJ/mol), Ors (−184,864.7 to −254,877.7 kJ/mol), CEHs (−212,819.6 to −279,727.0 kJ/mol), and CSPs (−56,539.6 to 74,956.7 kJ/mol). These outcomes reveal that power minimization successfully improves structural accuracy, making the proteins extra appropriate for downstream purposes like molecular docking. Moreover, correct energetic web site prediction for every protein ensures that docking targets the proper binding areas, enhancing the reliability of the outcomes. The Energetic web site residues of the proteins- COX1, AGI, ORs, CEHs, and CSPs to carry out docking research had been represented in (Desk 1). Collectively, the above steps ensured extra exact receptor-ligand interactions in additional docking research.
Protein-ligand docking
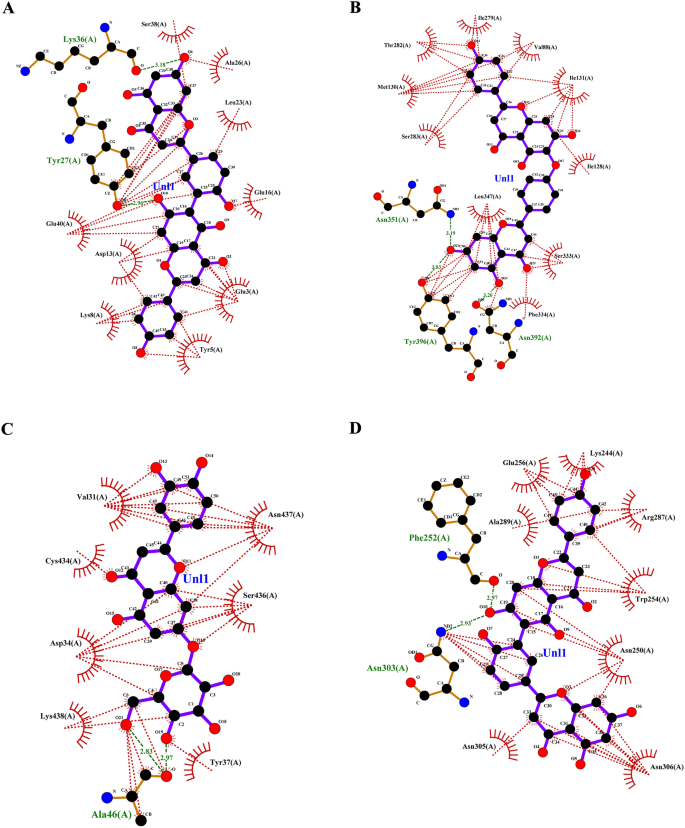
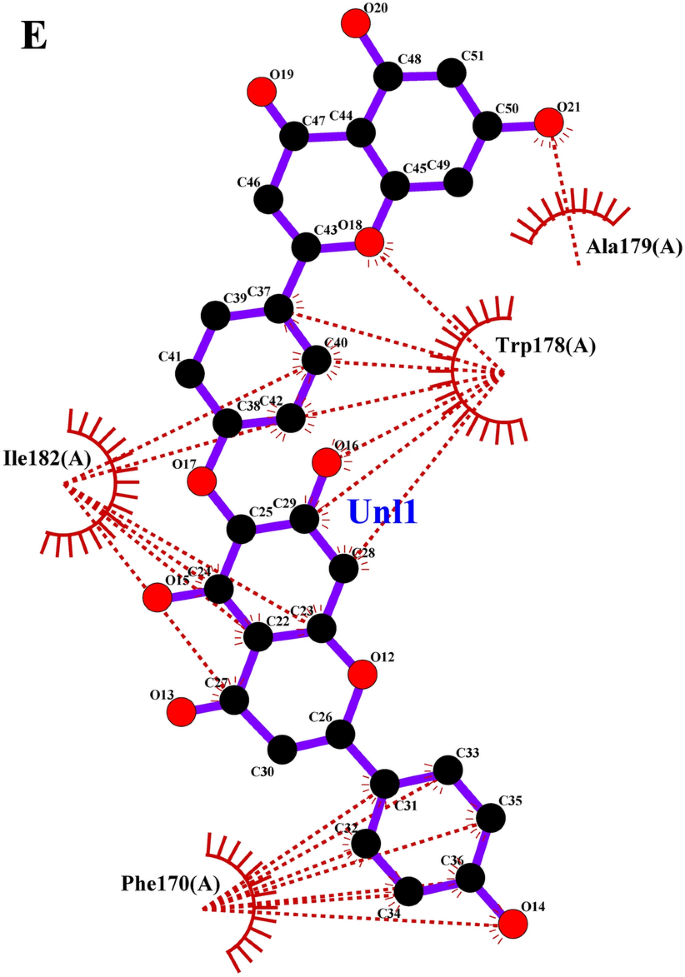
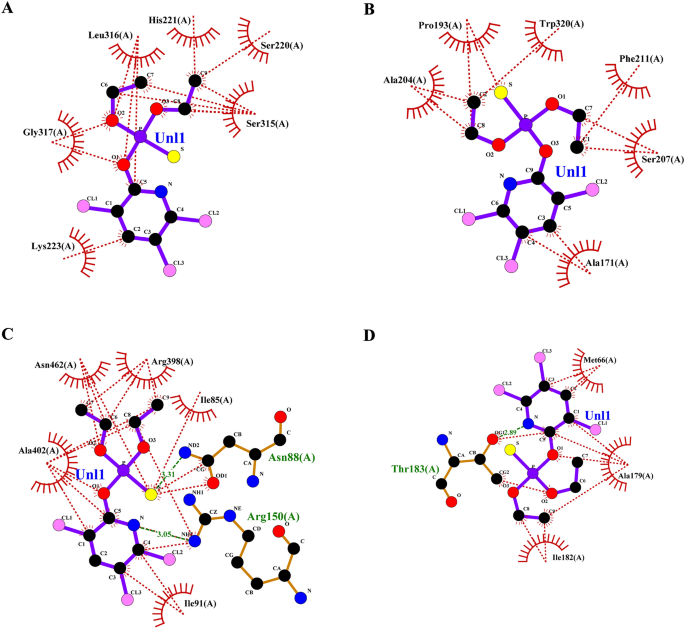
Focusing on invasive pest proteins with plant important oils or bioactive compounds affords a sustainable strategy to pest management. The method begins with deciding on key pest proteins important for survival or replica, comparable to enzymes or neural receptors. Bioactive compounds from important oils had been screened for his or her means to bind and inhibit these proteins. Protein-ligand docking and molecular dynamics simulations assist predict and validate the effectiveness of those interactions. This technique can result in the event of eco-friendly bioinsecticides that disrupt pest biology, decreasing crop harm whereas being protected for the atmosphere. From the docking research, the binding affinities of varied compounds of lemongrass (Desk 2) with S. litura proteins- COX1, AGI, ORs, CEHs, and CSPs had been noticed. Among the many examined compounds, cynaroside reveals the best binding affinity throughout most proteins, with a robust interplay of −37.656 kJ/mol for COX1, −37.2376 kJ/mol for CEHs, and −33.472 kJ/mol for each AGI and ORs, indicating its potent inhibitory potential. Equally, chamazulene reveals notable affinity for Ors (−35.9824 kJ/mol), making it one other promising compound for focusing on the ORs, a key modulator in insect nervous techniques. Moreover, spathulenol additionally reveals a robust binding affinity in direction of COX1 (−34.3088 kJ/mol), indicating the potential for inhibiting important metabolic pathways in S. litura. These compounds, notably cynaroside, may very well be promising candidates for growing bioinsecticides focusing on a number of physiological pathways in pests. From the cedarwood compounds docking research (Desk 3) with the identical proteins talked about above had been noticed, amentoflavone reveals the strongest binding affinity with CEHs (−45.1872 kcal/mol) and COX1 (−44.7688 kcal/mol), indicating its potential as a potent inhibitor for these enzymes crucial to insect metabolic processes. Robustaflavone additionally shows excessive binding affinity, notably with AGI (−42.6768 kcal/mol) and CEHs (−41.0032 kcal/mol), suggesting its function in disrupting digestive and metabolic features in S. litura. Hinokiflavone reveals sturdy binding to CEHs (−44.7688 kcal/mol) and ORs (−40.5848 kcal/mol), making it a possible candidate for interfering with the insect’s nervous and metabolic techniques. Moreover, cupressuflavone demonstrates appreciable affinity for AGI (−38.0744 kcal/mol) and Ors (−40.1664 kcal/mol), additional supporting its function as an efficient inhibitor of those goal websites. These compounds, particularly amentoflavone and robustaflavone, present sturdy multi-target exercise and are promising for the event of bioinsecticides in opposition to S. litura. One of the best ligand compounds from lemongrass and cedarwood important oils primarily based on their binding affinity with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs had been talked about in (Desk 4). The 2D interplay profile of greatest ligand compounds from lemongrass and cedarwood with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs from the ligplot plus had been represented in Fig. 4 (A, B, C, D, E, F, G, H, I & J). Docking interplay profile- residues of S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs with consolidated greatest binding affinity ligand compounds from lemongrass and cedarwood; and likewise, with reference molecule chlorpyrifos had been represented in (Desk 5). From the evaluation, lemongrass and cedarwood reveal a stronger and extra various interplay profile with the analyzed proteins in comparison with chlorpyrifos, as evidenced by their means to type a better variety of each hydrogen bonds and hydrophobic interactions. As an example, lemongrass varieties hydrogen bonds with key residues in COX1 (GLU 234, ALA 179, THY 183), CEHs (TYR 139, HIS 443), and CSPs (TYR 9, LYS 8), suggesting its potential for stabilizing protein-ligand complexes by way of polar interactions. Moreover, cedarwood continuously reveals hydrogen bonding with crucial residues in proteins comparable to AGI (ARG 366, HIS 362), additional enhancing its binding affinity. Hydrophobic interactions, important for enhancing ligand binding in non-polar environments, are additionally extra distinguished for lemongrass and cedarwood throughout a number of proteins, comparable to their interactions with residues like ILE 67, MET 66, and PRO 233 in COX1 and LEU 206 and TYR 424 in ORs. In distinction, chlorpyrifos reveals restricted hydrogen bond interactions and varieties fewer hydrophobic interactions with protein residues, which may restrict its stability and affinity throughout the binding websites. This implies that lemongrass and cedarwood, as a result of their richer interplay profiles, could exhibit higher efficacy in modulating protein exercise and may very well be extra appropriate candidates for bioactive or insecticidal purposes in comparison with chlorpyrifos in opposition to S. litura.
(A–J) The 2D interplay of greatest ligand compounds from lemongrass and cedarwood with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs utilizing ligplot plus. (A) The 2D interplay of compound spathulenol from lemongrass with chemosensory protein of S. litura. (B) The 2D interplay of compound robustaflavone from cedarwood with chemosensory protein of S. litura. (C) The 2D interplay of compound chamazulene from lemongrass with AGI of S. litura. (D) The 2D interplay of compound robustaflavone from cedarwood with AGI of S. litura. (E) The 2D interplay of compound cynaroside from lemongrass with octopamine protein of S. litura. (F) The 2D interplay of compound amentoflavone from cedarwood with octopamine protein of S. litura. (G) The 2D interplay of compound cynaroside from lemongrass with CEHs of S. litura. (H) The 2D interplay of compound hinokiflavone from cedarwood with CEHs of S. litura. (I) The 2D interplay of compound cynaroside from lemongrass with cytochrome c oxidase of S. litura. (J) The 2D interplay of compound hinokiflavone from cedarwood with cytochrome c oxidase of S. litura.
Grouped ligand-protein docking
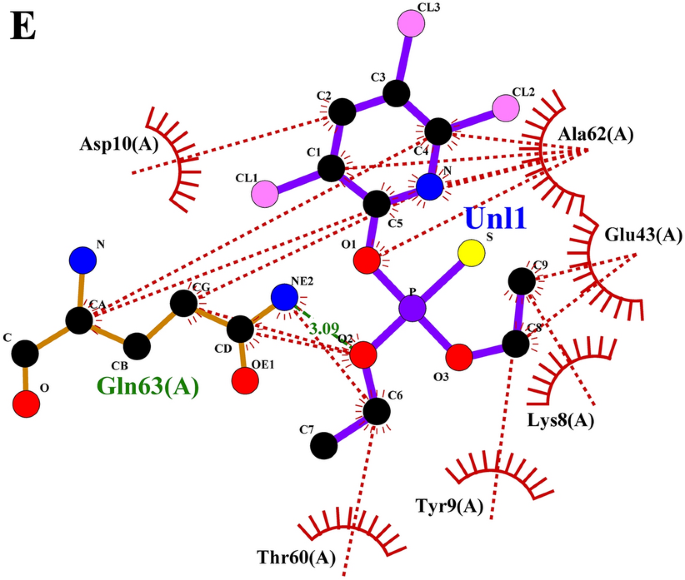
Later the grouped docking evaluation of one of the best binding affinity ligands from lemongrass and cedarwood with key S. litura proteins reveals vital interactions which will improve the understanding of bioinsecticide improvement. The mixed binding affinities point out promising synergistic results of the ligands. For the CSPs, the mix of spathulenol and robustaflavone yielded a binding affinity of −51.83976 kJ/mol, suggesting a robust interplay that might disrupt olfactory signaling within the pest. Within the case of AGI, chamazulene paired with robustaflavone demonstrated a binding affinity of −59.24544 kJ/mol, indicating efficient inhibition which will hinder carbohydrate metabolism. The ORs, essential for neuromodulation in bugs, confirmed a binding affinity of −56.6932 kJ/mol with the mix of cynaroside and amentoflavone, probably affecting the pest’s conduct. For CEHs, the mix of cynaroside and hinokiflavone achieved a binding affinity of −61.0864 kJ/mol, indicating a robust potential for enzyme inhibition. Lastly, the COX1 complicated exhibited a binding affinity of −46.98632 kJ/mol with cynaroside and hinokiflavone, which can impair power manufacturing within the pest. These findings spotlight the potential of mixed important oil formulations to focus on a number of proteins in S. litura, providing a promising avenue for the event of efficient bioinsecticides. The 2D interplay profile of mixed ligand compounds from lemongrass and cedarwood with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs from the ligplot plus had been represented in (Fig. 5A–E). Desk 6, grouped ligand docking binding affinity of mixed compounds from lemongrass and cedarwood with a comparability of chlorpyrifos in opposition to S. litura proteins- COX1, AGI, ORs, CEHs, and CSPs had been represented. In evaluating the binding affinities of chlorpyrifos with lemongrass and cedarwood compounds, the pure combos exhibit considerably stronger interactions with numerous proteins, indicating their potential as efficient options. For the CSPs, spathulenol and robustaflavone present a binding affinity of −51.83976 kJ/mol, in comparison with chlorpyrifos at −19.2464 kJ/mol. Equally, chamazulene and robustaflavone bind extra successfully to AGI (−59.24544 vs. −20.92 kJ/mol), and cynaroside and amentoflavone present stronger binding to ORs s (−56.6932 vs. −23.4304 kJ/mol). For COX1 and CEHs, pure compounds exhibit larger affinities (−61.0864 and −46.98632 kJ/mol, respectively) in comparison with chlorpyrifos (−21.7568 and −20.0832 kJ/mol). These outcomes recommend that lemongrass and cedarwood compounds could supply stronger pest management whereas being safer options to chlorpyrifos. The 2D interplay profile of chlorpyrifos with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs from the ligplot plus had been represented in (Fig. 6A–E). Grouped docking interplay profile of S. litura proteins with consolidated best-binding affinity ligand compounds from lemongrass and cedarwood, the reference molecule chlorpyrifos had been represented in (Desk 7). From the Desk 7, pure compound combos (cynaroside + hinokiflavone, chamazulene + robustaflavone, spathulenol + robustaflavone, and cynaroside + amentoflavone) reveal a extra beneficial interplay profile than chlorpyrifos when docked with the important thing S. litura proteins: COX1, AGI, CEHs, ORs, and CSPs. These pure combos type stronger and extra quite a few interactions with crucial residues, enhancing their potential to bind and disrupt the operate of those proteins. For instance, cynaroside + hinokiflavone varieties sturdy hydrophobic interactions with residues like PHE 170, ILE 182, TRP 178, and ALA 179 in COX1, and with LEU 347, PHE 334, ILE 128, and ILE 131 in CEHs, suggesting a extra steady and efficient binding in comparison with chlorpyrifos, which varieties fewer interactions with residues like MET 66, ALA 179, and ILE 182. Equally, chamazulene + robustaflavone establishes vital hydrogen bonds with residues comparable to PHE 252 and ASN 303 in AGI, whereas chlorpyrifos reveals no hydrogen bonding with this enzyme, indicating a weaker potential for inhibiting the protein. The pure compound pair additionally interacts hydrophobically with residues like ALA 289, GLU 256, and TRP 254, additional enhancing its binding stability. Within the ORs, cynaroside + amentoflavone varieties a crucial hydrogen bond with ALA 46 and engages in hydrophobic interactions with residues comparable to TYR 37, LYS 438, and ASP 34, which may disrupt receptor signaling extra successfully than chlorpyrifos, which binds weakly to residues like ASN 88 and ARG 150. Lastly, spathulenol + robustaflavone demonstrates sturdy hydrogen bonds with LYS 36 and TYR 27 within the CSPs, and varieties hydrophobic contacts with key residues comparable to SER 38, LEU 23, and GLU 16, whereas chlorpyrifos reveals restricted interplay with residues like GLN 63. Total, the pure compounds exhibit extra in depth and stronger interactions with key residues throughout all proteins analyzed.
(A–E) 2D interplay profile of mixed ligand compounds from lemongrass and cedarwood with S. litura proteins—COX1, AGI, ORs, CEHs and CSPs utilizing ligplot plus. (A) The 2D interplay of compounds spathulenol + robustaflavone from lemongrass and cedarwood with chemosensory protein of S. litura. (B) The 2D interplay of compounds cynaroside + hinokiflavone from lemongrass and cedarwood with CEHs of S. litura. (C) The 2D interplay of compounds cynaroside + amentoflavone from lemongrass and cedarwood with octopamine receptor protein of S. litura. (D) The 2D interplay of compounds chamazulene + robustaflavone from lemongrass and cedarwood with AGI of S. litura. (E) The 2D interplay of compounds cynaroside + hinokiflavone from lemongrass and cedarwood with cytochrome c oxidase of S. litura.
(A–E) The 2D interplay profile of chlorpyrifos with S. litura proteins—COX1, AGI, ORs, CEHs, and CSPs from the ligplot plus. (A) The 2D interplay of compounds chlorpyrifos with cytochrome c oxidase of S. litura. (B) The 2D interplay of compounds chlorpyrifos with AGI of S. litura. (C) The 2D interplay of compounds chlorpyrifos with octopamine recrptor of S. litura. (D) The 2D interplay of compounds chlorpyrifos with CEHs and of S. litura. (E) The 2D interplay of compounds chlorpyrifos with cytochrome c oxidase of S. litura.
The comparative docking evaluation of pure compounds from lemongrass and cedarwood in opposition to key Spodoptera litura proteins revealed considerably larger binding affinities than the traditional insecticide chlorpyrifos, indicating their potential as efficient bioinsecticides. These findings align with prior research that explored the inhibitory results of important oil constituents on crucial insect biochemical pathways. Awad et al.48 demonstrated that terpinen-4-ol, (Z)-citral, and α-pinene, the first elements of marjoram, citronella, and rosemary important oils, exhibited sturdy binding affinities with glutathione S-transferase (GST), suggesting their potential to intrude with cleansing mechanisms in bugs. Equally, Aathmanathan et al.49 recognized spinosyn A and milbemycin A4 as potent inhibitors of the vitellogenin receptor (VgR), a key protein in reproductive processes, highlighting their function in disrupting pest fertility. The current research extends these findings by exhibiting that compounds comparable to spathulenol, robustaflavone, chamazulene, and cynaroside exhibited stronger and extra steady interactions with CSPs, AGI, and ORs than chlorpyrifos. Furthermore, the noticed hydrogen bonding and hydrophobic interactions with crucial residues reinforce the structural stability of those ligand-protein complexes, additional supporting the insecticidal efficacy of pure compounds. Collectively, these research underscore the potential of plant-derived bioactive molecules as eco-friendly options to artificial pesticides, providing focused mechanisms of pest management whereas mitigating environmental toxicity.
Simulation research
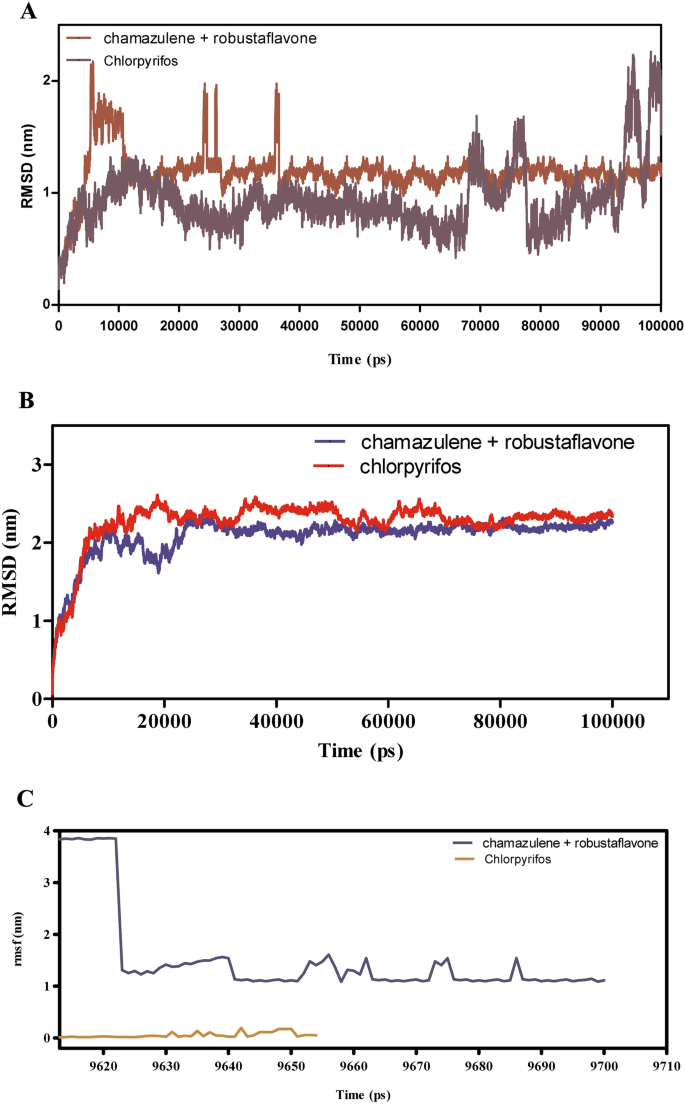
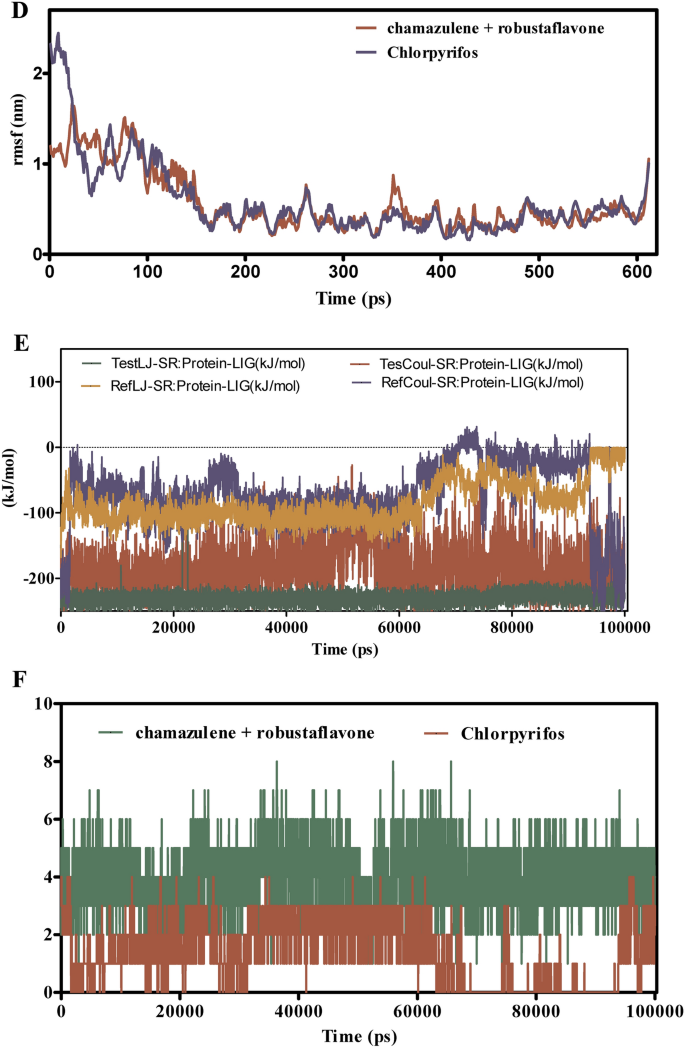
The molecular dynamics (MD) simulations offered in (Fig. 7A–F) consider the binding stability, flexibility, and interplay energies of chamazulene + robustoflavone versus chlorpyrifos with alpha-glucosidase over a 100 ns trajectory. The foundation imply sq. deviation (RMSD) evaluation in Fig. 7A reveals that chamazulene + robustoflavone maintains a median RMSD of roughly 1.5 Å, indicating a steady binding pose with minimal positional shifts. In distinction, chlorpyrifos reveals larger RMSD fluctuations round 2.0 Å, suggesting a much less steady interplay throughout the binding pocket. Correspondingly, Fig. 7B reveals that the RMSD of AGI itself stays regular at roughly 1.2 Å when certain to chamazulene + robustoflavone, in comparison with a median of 1.6 Å with chlorpyrifos, which often spikes. This means that chlorpyrifos induces extra conformational perturbations within the enzyme, probably compromising binding stability. Determine 7C demonstrates that chamazulene + robustoflavone reveals a low Root imply sq. fluctuation (RMSF) of round 1.5 Å or much less, reflecting minimal atomic motion and powerful retention throughout the binding web site. Chlorpyrifos, nevertheless, reveals larger RMSF values as much as 2.5 Å, indicating better flexibility and weaker interactions. Moreover, Fig. 7D highlights that AGI residues interacting with chamazulene + robustoflavone show decrease RMSF (~ 1.0 Å) in comparison with these with chlorpyrifos (~ 2.0 Å), additional supporting the notion of a extra stabilized enzyme construction upon binding with chamazulene + robustoflavone. The interplay power evaluation in Fig. 7E reveals that chamazulene + robustoflavone varieties extra favorable Lennard–Jones and coulomb interactions, with coulomb energies averaging round −150 kJ/mol, in comparison with chlorpyrifos’s -100 kJ/mol. This signifies stronger electrostatic points of interest facilitating efficient binding. Determine 7F reveals that chamazulene + robustoflavone constantly varieties 3–5 hydrogen bonds with bond lengths round 2.0 Å, whereas chlorpyrifos varieties fewer (1–2) and longer, extra variable hydrogen bonds (2.5–3.0 Å). The strong hydrogen bonding community of chamazulene + robustoflavone contributes considerably to its superior binding stability and affinity. Collectively, chamazulene + robustoflavone reveal enhanced binding stability, decreased flexibility, stronger interplay energies, and extra steady hydrogen bonding with AGI in comparison with chlorpyrifos. These traits underscore the potential of chamazulene + robustoflavone as a simpler inhibitor of AGI.
(A–F) The molecular dynamic simulations of AGI with ligands- chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns. The Root imply sq. deviation (RMSD), Root imply sq. fluctuation (RMSF), interplay energies, and Hydrogen bond interactions of ligands- chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns had been represented. (A) Root imply sq. deviation (RMSD) of ligands- chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns. (B) Root imply sq. deviation (RMSD) of AGI interplay with chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns. (C) Root imply sq. fluctuation (RMSF) of ligands chamazulene + robustoflavone and chlorpyrifos (reference) interplay with receptor for 100 ns. (D) Root imply sq. fluctuation (RMSF) of AGI interplay with chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns. (E) Lennard–Jones interactions and Coulomb interactions energies of AGI receptor and ligands- chamazulene + robustoflavone and chlorpyrifos (reference) for 100 ns. (F) Hydrogen bond interactions of chamazulene + robustoflavone and chlorpyrifos (reference) with AGI receptor for 100 ns.
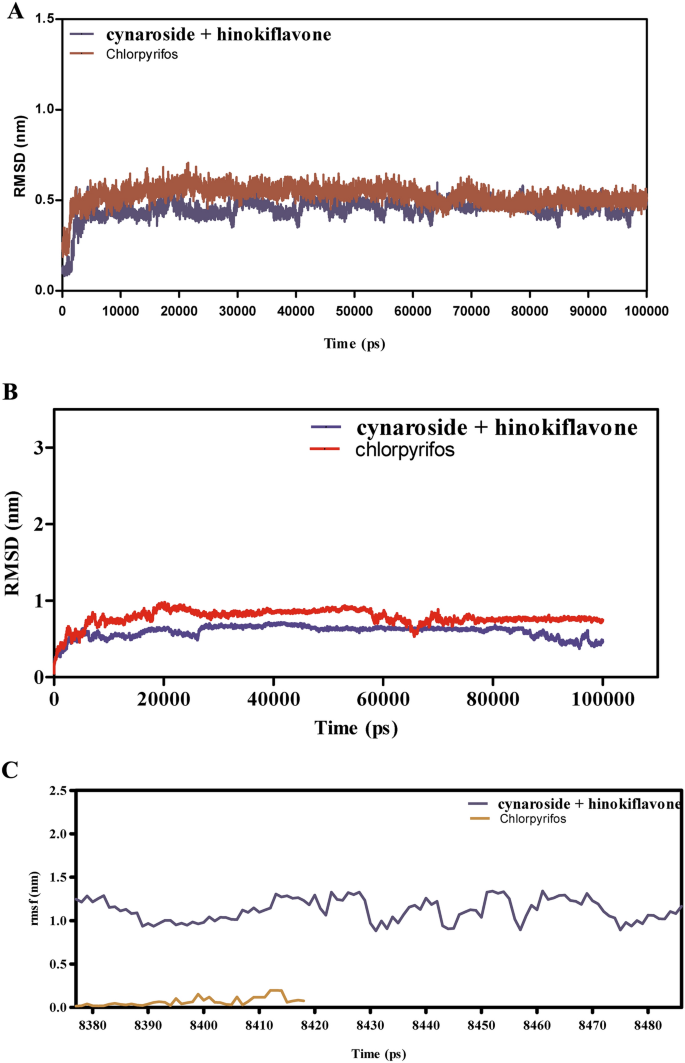
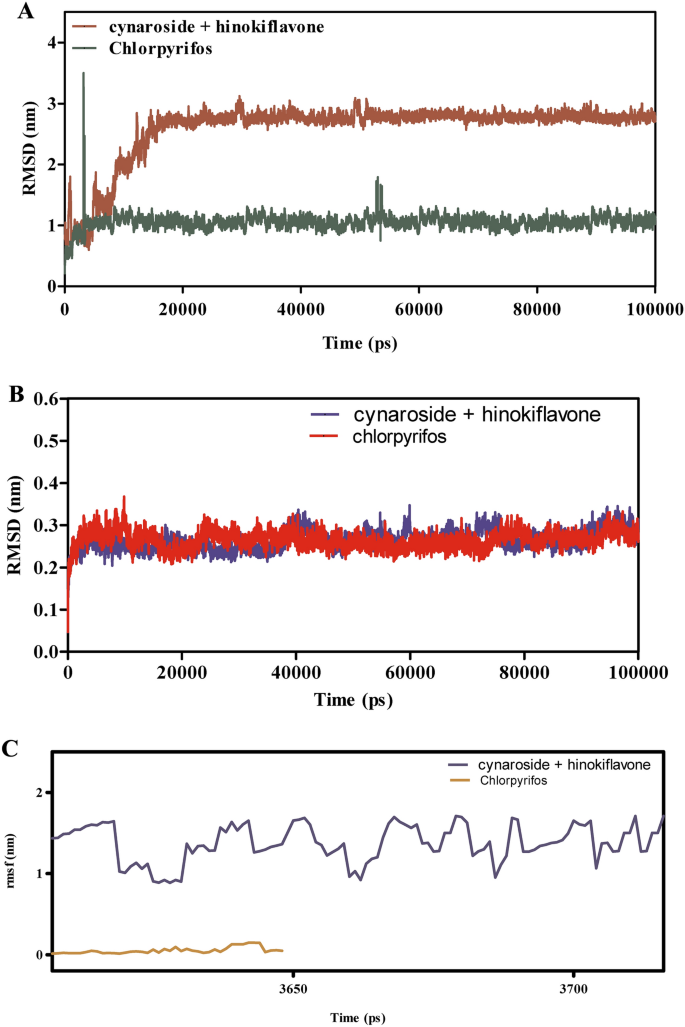
Determine 8A–F explores the binding dynamics of cynaroside + hinkoflavone versus chlorpyrifos with CEHs over a 100 ns simulation. RMSD analyses in Fig. 8A,B point out that cynaroside + hinkoflavone maintains steady RMSD values between 1.5–2.0 Å, suggesting tight binding and minimal structural deviation. In distinction, chlorpyrifos reveals better RMSD variability, indicative of weaker and fewer steady binding. Moreover, when certain to COX1, cynaroside + hinkoflavone leads to a managed RMSD peaking round 2.2 Å, whereas chlorpyrifos induces wider fluctuations, implying a much less steady interplay. Determine 8C reveals that cynaroside + hinkoflavone reveals decrease RMSF values, usually round 1.0 Å, indicating decreased atomic flexibility and stronger anchoring throughout the binding web site. Conversely, chlorpyrifos shows RMSF values exceeding 1.5 Å, suggesting larger residue mobility and fewer inflexible binding. Determine 8D additional helps this by exhibiting that residues interacting with cynaroside + hinkoflavone have decrease fluctuations, sustaining the protein’s structural integrity. The interplay power profile in Fig. 8E reveals that cynaroside + hinkoflavone varieties extra favorable and steady Lennard–Jones and Coulomb interactions in comparison with chlorpyrifos, which reveals erratic power readings indicative of inconsistent binding. Determine 8F illustrates that cynaroside + hinkoflavone constantly varieties extra hydrogen bonds all through the simulation, enhancing the soundness of the ligand–protein complicated. In distinction, chlorpyrifos varieties fewer and fewer steady hydrogen bonds, correlating with its weaker binding profile. Cynaroside + hinkoflavone outperforms chlorpyrifos when it comes to binding stability, decreased flexibility, favorable interplay energies, and strong hydrogen bonding with CEHs. These findings place cynaroside + hinkoflavone as a simpler ligand for inhibiting CEHs.
(A–F) The molecular dynamic simulations of CEHs with ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. The Root imply sq. deviation (RMSD), Root imply sq. fluctuation (RMSF), interplay energies, and Hydrogen bond interactions of ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns had been represented. (A) Root imply sq. deviation (RMSD) of ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (B) Root imply sq. deviation (RMSD) of CEHs interplay with cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (C) Root imply sq. fluctuation (RMSF) of ligands cynaroside + hinkoflavone and chlorpyrifos (reference) interplay with receptor for 100 ns. (D) Root imply sq. fluctuation (RMSF) of CEHs interplay with cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (E) Lennard–Jones interactions and Coulomb interactions energies of CEHs receptor and ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (F) Hydrogen bond interactions of cynaroside + hinkoflavone and chlorpyrifos (reference) with CEHs receptor for 100 ns.
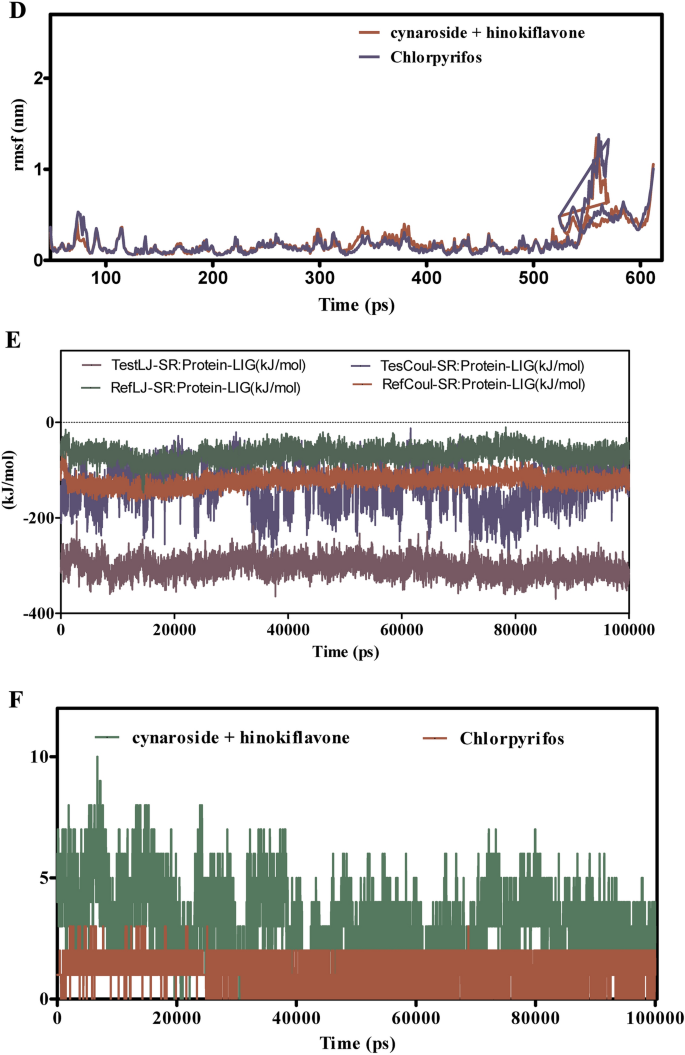
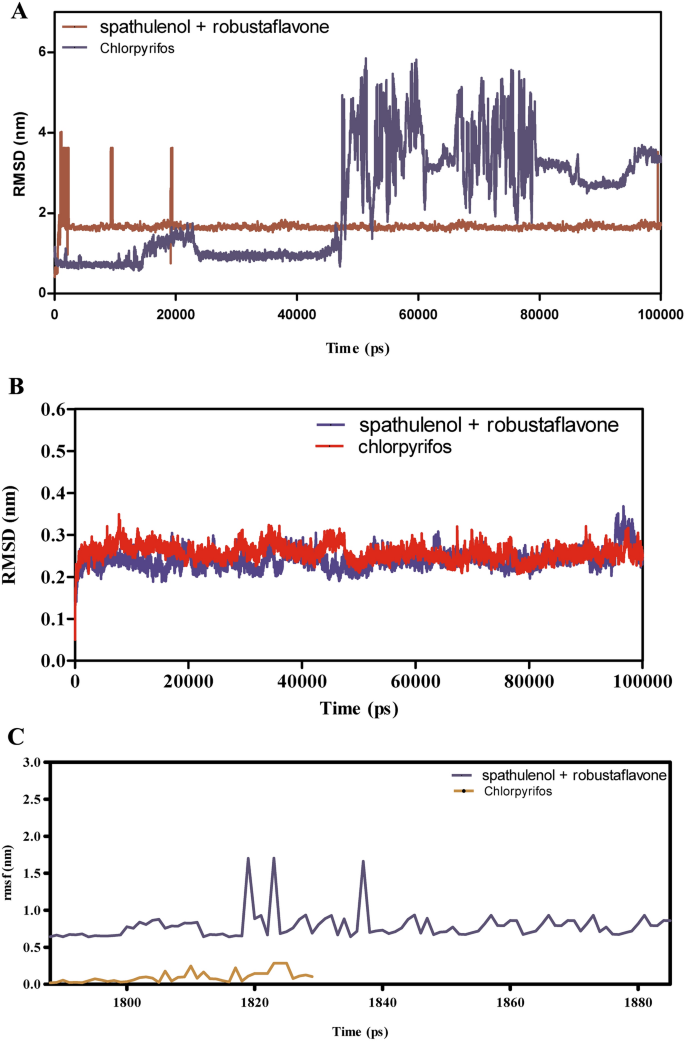
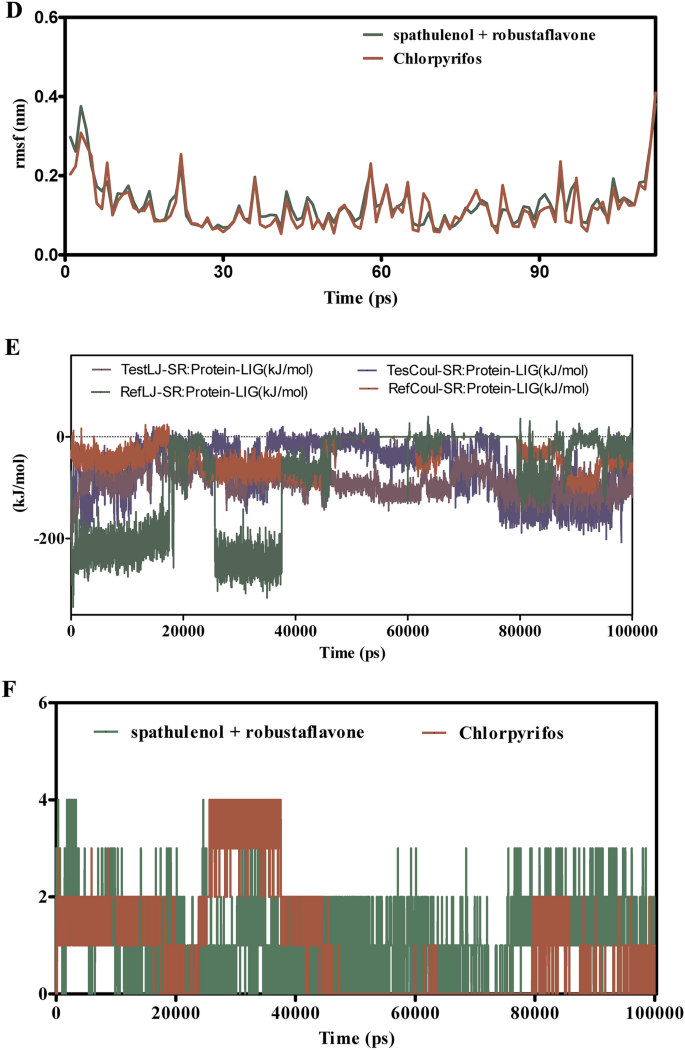
Determine 9A–F presents the MD simulation outcomes evaluating spathulenol + robustaflavone with chlorpyrifos of their interactions with CSPs over 100 ns. The RMSD evaluation in Fig. 9A,B signifies that the spathulenol + robustaflavone complicated maintains RMSD values between 1.5 to 2.5 Å, reflecting a steady binding mode. In distinction, chlorpyrifos complexes exhibit larger RMSD values starting from 2.0 to three.5 Å, suggesting better structural variations and fewer steady binding. Determine 9C reveals that the RMSF values for key binding web site residues within the spathulenol + robustaflavone complicated stay between 1.0 to 1.8 Å, indicating restricted flexibility and a extra inflexible binding conformation. Conversely, chlorpyrifos-bound complexes show RMSF values between 1.5 to 2.3 Å, indicating elevated residue flexibility and probably weaker interactions. Interplay energies depicted in Fig. 9E reveal that spathulenol + robustaflavone varieties stronger Lennard–Jones interactions (−15 to −25 kcal/mol) and extra favorable Coulomb energies (−10 to −15 kcal/mol) in comparison with chlorpyrifos (−10 to −20 kcal/mol for Lennard–Jones and −8 to −12 kcal/mol for Coulomb). Determine 9F illustrates that spathulenol + robustaflavone constantly varieties extra steady hydrogen bonds with bond distances between 2.8 to three.2 Å, whereas chlorpyrifos varieties fewer and extra transient hydrogen bonds with longer distances (3.0 to three.5 Å). Spathulenol + robustaflavone reveals superior binding stability, decreased flexibility, stronger interplay energies, and extra steady hydrogen bonding with CSPs in comparison with chlorpyrifos. These attributes recommend that spathulenol + robustaflavone varieties a simpler and steady ligand-protein complicated.
(A–F) The molecular dynamic simulations of CSPs with ligands- spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns. The Root imply sq. deviation (RMSD), Root imply sq. fluctuation (RMSF), interplay energies, and Hydrogen bond interactions of ligands- spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns had been represented. (A) Root imply sq. deviation (RMSD) of ligands- spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns. (B) Root imply sq. deviation (RMSD) of CSPs interplay with spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns. (C) Root imply sq. fluctuation (RMSF) of ligands spathulenol + robustaflavone and chlorpyrifos (reference) interplay with receptor for 100 ns. (D) Root imply sq. fluctuation (RMSF) of CSPs interplay with spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns. (E) Lennard–Jones interactions and Coulomb interactions energies of CSPs receptor and ligands- spathulenol + robustaflavone and chlorpyrifos (reference) for 100 ns. (F) Hydrogen bond interactions of spathulenol + robustaflavone and chlorpyrifos (reference) with CSPs receptor for 100 ns.
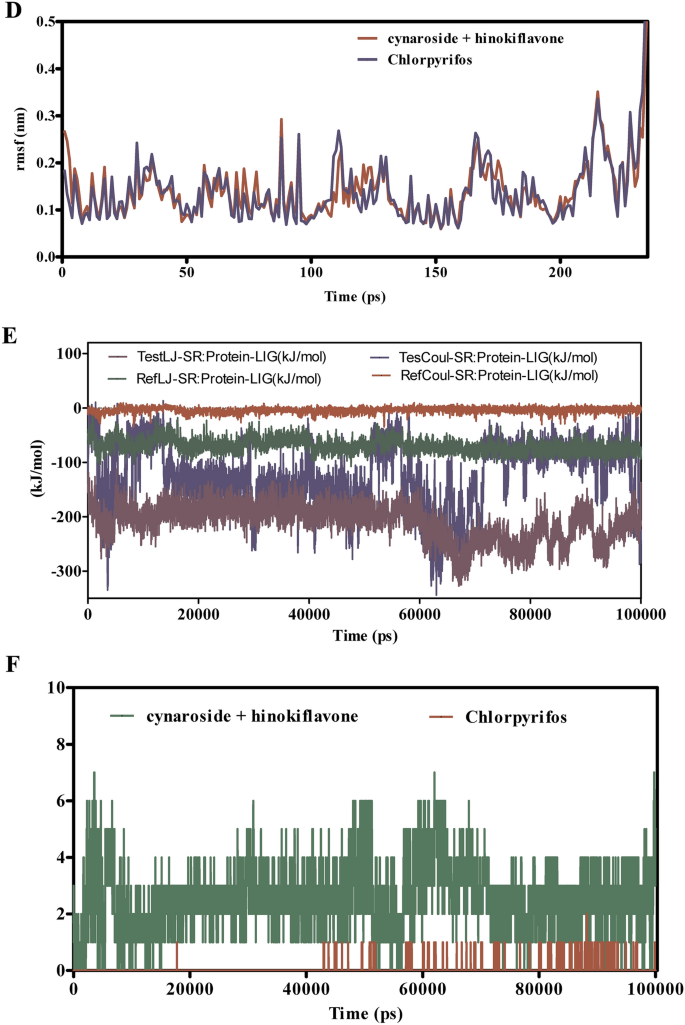
Determine 10A–F examines the interactions of cymaroside + amentoflavone versus chlorpyrifos with COX1 over a 100 ns simulation interval. The RMSD profiles in Fig. 10A,B point out that cymaroside + amentoflavone maintains steady deviation values between 2.5 to three.2 Å, suggesting constant binding. In distinction, chlorpyrifos reveals larger RMSD fluctuations starting from 3.0 to 4.5 Å, indicative of a much less steady binding configuration throughout the enzyme’s energetic web site. Determine 10C demonstrates that residues throughout the binding web site interacting with cymaroside + amentoflavone exhibit RMSF values between 1.2 to 1.8 Å, reflecting decreased flexibility and a extra inflexible binding conformation. Conversely, chlorpyrifos-bound complexes show larger RMSF values (1.5 to 2.5 Å), indicating elevated residue mobility and probably destabilizing interactions. Interplay power evaluation in Fig. 10E reveals that cymaroside + amentoflavone varieties stronger Lennard–Jones and Coulombic interactions (−150 to −180 kJ/mol) in comparison with chlorpyrifos (−100 to −130 kJ/mol), suggesting a extra energetically beneficial and steady binding. Determine 10F reveals that cymaroside + amentoflavone constantly varieties 2 to 4 steady hydrogen bonds with distances of two.8 to three.2 Å, whereas chlorpyrifos varieties fewer and fewer constant hydrogen bonds with longer distances (3.2 to three.6 Å). Cymaroside + amentoflavone demonstrates enhanced binding stability, decreased flexibility, stronger interplay energies, and extra steady hydrogen bonding with COX1 in comparison with chlorpyrifos. These findings recommend that cymaroside + amentoflavone varieties a simpler and steady ligand–protein complicated.
(A–F) The molecular dynamic simulations of COX1 with ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. The Root imply sq. deviation (RMSD), Root imply sq. fluctuation (RMSF), interplay energies, and Hydrogen bond interactions of ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns had been represented. (A) Root imply sq. deviation (RMSD) of ligands- cymaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (B) Root imply sq. deviation (RMSD) of COX1 interplay with cymaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (C) Root imply sq. fluctuation (RMSF) of ligands cymaroside + hinkoflavone and chlorpyrifos (reference) interplay with receptor for 100 ns. (D) Root imply sq. fluctuation (RMSF) of COX1 interplay with cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (E) Lennard–Jones interactions and Coulomb interactions energies of COX1 receptor and ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 100 ns. (F) Hydrogen bond interactions of cynaroside + hinkoflavone and chlorpyrifos (reference) with COX1 receptor for 100 ns.
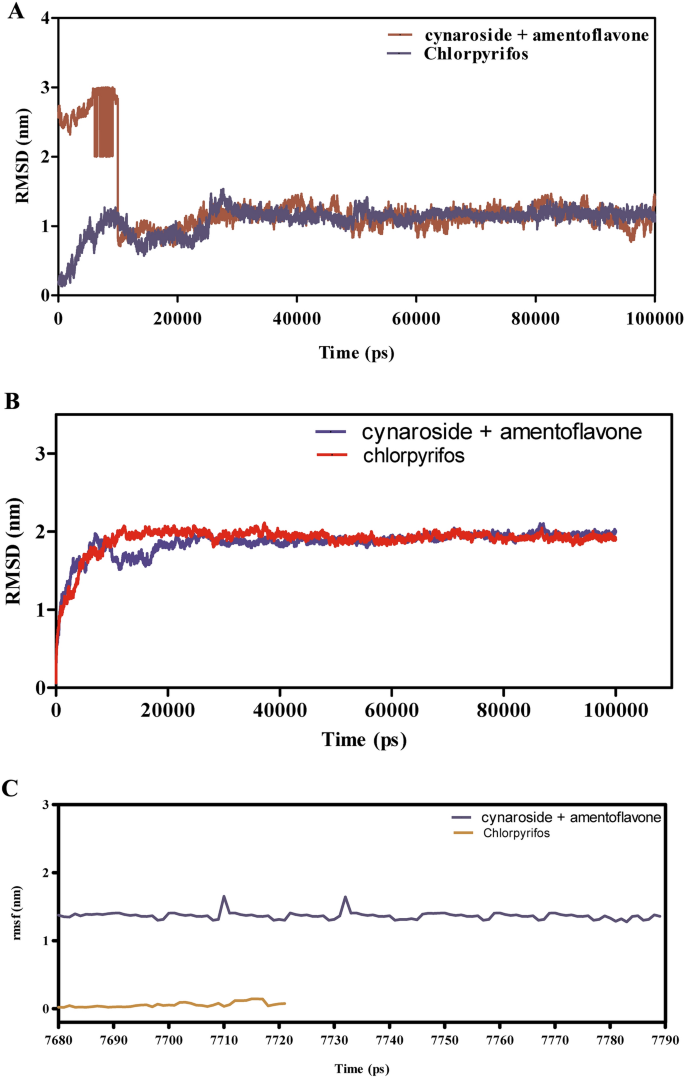
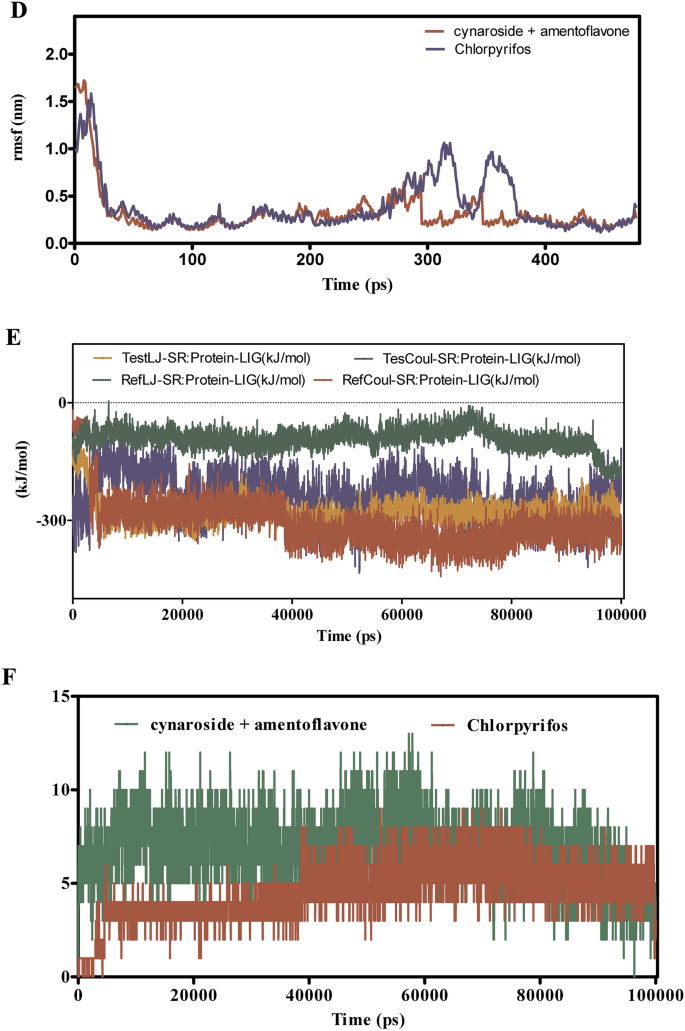
Determine 11A–F presents the MD simulation outcomes for the interactions between cymaroside + amentoflavone and chlorpyrifos with the ORs over a 110 ns trajectory. The RMSD evaluation in Fig. 11A signifies that cymaroside + amentoflavone maintains RMSD values fluctuating round 1.5–2.5 Å, reflecting a steady ligand construction. In distinction, chlorpyrifos reveals larger RMSD deviations, suggesting extra pronounced conformational adjustments. Determine 11B reveals that the ORs -ligand complicated with cymaroside + amentoflavone maintains a smoother RMSD profile (1–2 Å) in comparison with chlorpyrifos, which reaches as much as 3 Å, indicating weaker binding stability.
(A–F) The molecular dynamic simulations of ORs with ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 110 ns. The Root imply sq. deviation (RMSD), Root imply sq. fluctuation (RMSF), interplay energies, and Hydrogen bond interactions of ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 110 ns had been represented. (A) Root imply sq. deviation (RMSD) of ligands- cymaroside + amentoflavone and chlorpyrifos (reference) for 110 ns. (B) Root imply sq. deviation (RMSD) of ORs interplay with cymaroside + amentoflavone and chlorpyrifos (reference) for 110 ns. (C) Root imply sq. fluctuation (RMSF) of ligands cymaroside + amentoflavone and chlorpyrifos (reference) interplay with receptor for 110 ns. (D) Root imply sq. fluctuation (RMSF) of ORs interplay with cynaroside + hinkoflavone and chlorpyrifos (reference) for 110 ns. (E) Lennard–Jones interactions and Coulomb interactions energies of ORs receptor and ligands- cynaroside + hinkoflavone and chlorpyrifos (reference) for 110 ns. (F) Hydrogen bond interactions.
Determine 11C,D illustrate that the RMSF values for receptor residues interacting with cymaroside + amentoflavone stay between 0.5–1.5 Å, indicating restricted flexibility and a extra inflexible binding conformation. Conversely, chlorpyrifos induces larger RMSF values exceeding 2 Å in sure areas, suggesting elevated native flexibility and probably much less steady binding interactions. Determine 11E reveals that cymaroside + amentoflavone varieties stronger Lennard–Jones and Coulomb interactions inside atomic distances (1–3 Å) in comparison with chlorpyrifos, which reveals weaker interplay profiles. Determine 11F highlights that cymaroside + amentoflavone constantly varieties extra frequent and steady hydrogen bonds inside an optimum distance vary (1.5–2.5 Å), whereas chlorpyrifos varieties fewer and extra transient hydrogen bonds with longer distances, indicating weaker interplay stability. Cymaroside + amentoflavone reveals superior binding stability, decreased receptor flexibility, stronger interplay energies, and extra steady hydrogen bonding with the ORs in comparison with chlorpyrifos. These outcomes recommend that cymaroside + amentoflavone is a simpler and steady ligand for focusing on the ORs.
Throughout all studied proteins: AGI, CEHs, CSPs, COX1, and the Ors, various ligand combos (chamazulene + robustoflavone, cynaroside + hinkoflavone, spathulenol + robustaflavone, and cymaroside + amentoflavone) constantly reveal superior binding stability, decreased flexibility, stronger interplay energies, and extra steady hydrogen bonding in comparison with the reference compound chlorpyrifos. These findings collectively spotlight the potential of those pure ligand combos as simpler and steady options to chlorpyrifos in modulating goal protein features. The improved binding traits of the choice ligand combos recommend their potential as viable substitutes or complementary brokers to chlorpyrifos, notably in purposes requiring steady and particular protein interactions. Future research ought to concentrate on experimental validation of those computational findings, exploring the organic efficacy and security profiles of those ligands in related organic techniques. Moreover, additional optimization of those ligand buildings may result in the event of extremely potent inhibitors with minimal opposed results, contributing to safer and simpler therapeutic methods.