Motivation
HPV is a standard sexually transmitted an infection that may be implicated within the formation of assorted most cancers sorts, together with cervical, vulvar, vaginal, anal, penile, and oropharyngeal cancers, in addition to genital warts1 GARDASIL®9 has considerably aided prevention of HPV-related illnesses. Scientific trials have demonstrated the efficacy of GARDASIL®9 in stopping infections and illnesses,1 resulting in its contribution in direction of lowering HPV-related morbidity and mortality. Demand for HPV vaccination is predicted to proceed to climb notably involving a necessity to extend vaccine protection and accessibility in much less developed areas2.
The World Well being Group (WHO) has articulated an pressing want round technological improvements for vaccines, together with modelling, to handle storage situation administration to extend affected person entry, enhance vaccine efficiency, decrease value, and improve flexibility3 Warmth stability, cold-chain provide administration, and resultant avoidable wastage are main points in deployment of vaccines to decrease and center revenue international locations3,4 An space of explicit significance is to handle data uncertainty in understanding temperature limitations of vaccines5 Predictive stability and shelf-life prediction for brand spanking new pharmaceutical product batches are believed to play a job within the objective to speed up affected person entry of present and future vaccines6.
Traditionally, Q1/Q5C have offered restricted steerage for the way a lot extrapolation is acceptable for figuring out shelf life whereas specializing in easy regression evaluation in setting shelf-life. The extrapolation strategies described are restricted in scope, and by definition will not be capable of incorporate multi-factor evaluation past a given development at a given set of circumstances. To handle these deficiencies, proposals in direction of incorporation of enhanced stability modelling7 have been endorsed by the Worldwide Council for Harmonisation of Technical Necessities for Prescribed drugs for Human Use (ICH)8 for inclusion within the trade pointers. The ICH pointers Q1A/Q5C are present process revision to incorporate an annex for stability modelling and model-informed shelf-life setting inclusive of Bayesian statistics and different complicated fashions that take into consideration a number of components of stability-indicating data. Prediction intervals described on this work can modify for a number of variables and situations and affirm the continued validity of developments. Therefore the outlined method shouldn’t be solely aligned with the evolving regulatory panorama, but in addition particularly highlights the hole that the rule of thumb replace will mirror.
Actual-time pharmaceutical stability research in assist of shelf-life and regulatory submissions are time-limiting and resource-intensive in biopharmaceutical analysis and growth. Strategies to foretell long-term stability in an effort to speed up time to deployment to sufferers of novel biopharmaceutical medicines and vaccines are of nice curiosity to industrial, authorities, and patient-centric stakeholders. The aspirational state of predictive stability strategies is to allow fast affected person entry to new medicines and vaccines by precisely predicting and establishing drug product expiry/shelf-life quicker and with fewer sources. The purpose is to show that this future technique will be achieved by leveraging lower than the traditionally acceptable quantity of stability experimental information throughout modalities, whereas sustaining the identical confidence of a product assembly crucial high quality attributes by shelf-life in regulatory filings. It is usually of curiosity to cut back reliance on massive formal stability research to handle adjustments in e.g. product, course of, container-closure elements, and many others. This may serve the objective of quicker time to the clinic for sufferers in want and extra fast entry to new cutting-edge therapies.
Purposes of predictive stability for pharmaceutical shelf-life
Stability is crucial to product high quality and pivotal in direction of regulatory expectations from well being authorities. As stability is crucial all through the complete pharmaceutical growth and commercialization panorama, and the understanding thereof evolves in the course of the product lifecycle, predictive stability applied sciences and methodologies maintain monumental potential for saving vital time in addition to human and materials sources. Within the context of better future adoption and acceptance of such strategies, the utilization of those instruments may result in a lower within the workload related to experimental testing all through the assorted complicated levels, encompassing materials manufacturing, pattern preparation, stability testing at a number of time factors, information acquisition, evaluation and reporting, formal documentation creation, and extra research performed all through the length of a product’s lifespan. Whereas mannequin validation research would nonetheless be wanted, together with the expectation round formal real-time information, predictive applied sciences are helpful to speed up growth of latest medicines, cut back experimental testing, and leveraging model-informed threat administration.
Among the advantages of predictive stability are elucidated: (1) Predict stability profiles throughout circumstances & time factors primarily based on a smaller experimental information set (for instance, use short-term and/or accelerated stability research to foretell long-term shelf-life) together with for in-use interval, day out of refrigeration, and vial monitor labels. (2) Leverage historic prior platform information for molecular attributes for related assemble or analogous molecular household. (3) Inform threat posture round product liabilities (low, medium, and excessive dangers) for proactive identification and improved administration of dangers. (4) Construct elementary understanding of the physicochemical degradation pathways main in direction of instability versus stability of latest molecular entities. (5) Display and optimize product compositions extra shortly by higher characterizing and projecting the function of excipients in enhancing or suppressing total stability whereas figuring out the circumstances that produce the best total stability profile. (6) Quantitatively interrogate the connection of co-factors like course of circumstances, container-closure methods, and many others. onto stability profiles. (7) Higher tackle course of and analytical variability and interrogate/deconvolute these components in relation to stability developments. (8) Enhance stability mannequin prediction confidence and statistically rationalize outliers inside the vary of mannequin variance. (9) Enhance predictability on industrial manufacture robustness and the suggestions loop to drug discovery. (10) Apply superior statistical and machine studying algorithms to establish composition and course of variables more likely to require strict controls in an effort to obtain constant stability all through the product lifecycle.
It’s identified that compounds with fewer molecular legal responsibility dangers have better total physicochemical stability resulting in greater chance of success of finishing scientific trials and changing into industrial merchandise,9 therefore new strategies to foretell stability or validate intrinsic molecular dangers can be of great profit. To facilitate risk-based scientific evaluation of stability the means to higher leverage predictive stability is a subject below in depth dialogue10 Business is especially considering increasing software of predictive stability approaches to massive molecule merchandise like vaccines and biologics as these domains are rising as a proportion of the trade’s total portfolio of modalities. In the direction of this objective, establishments wish to broaden purposes of assorted predictive and stability modelling approaches11,12.
Present approaches
Important progress has been made in predictive stability for small-molecules. Regulatory acceptance has been achieved within the small-molecule area10 for setting preliminary shelf-life, preliminary retest interval, shelf-life of a formulation variant, justifying storage circumstances for drug substance (DS) from a brand new course of, and gauging affect of drug product (DP) course of adjustments on shelf-life. Traditionally purposes within the large-molecule area have lagged the small-molecule area, but synthetically or biologically derived massive molecules are assuming an rising share of the general biopharmaceutical pipeline. However, vital progress in massive molecule predictive stability has been remodeled the previous a number of years. Investigations in predictive stability for giant molecules have been targeted on experimental approaches,13,14,15,16 excipient17 and adjuvant18 pushed interactions, and particular mechanisms like aggregation-driven behaviour,19,20 in addition to enhancements to preliminary discovery developability assessments and methodologies21,22.
A complete evaluation of stability-impacting components is crucial for any predictive mannequin. A large number components and their relative significance should be thought of. For instance, it was proven that small adjustments in conformational construction and colloidal stability of a given formulation might not essentially be predictive of long-term instability of excessive molecular weight species formation (aggregation)23 Furthermore, stability additionally must be assessed in a holistic context (e.g., past change in efficiency, aggregation, and many others.) in relation to different key manufacturing and scientific concerns. For instance, it was proven that totally different combos of antigen and adjuvants in a vaccine candidate may lead to a greater stability profile whereas concurrently leading to suboptimal immune responses24 Thus modelling strategies are greatest adopted when they’re executed with shut integration to experimental strategies25 and within the context of broader biopharmaceutical manufacturing methodologies26,27,28,29.
Massive-molecule adoption of predictive stability within the biopharmaceutical growth context has been proposed for biologics30 and vaccines31 alongside a common modelling method32. As a result of the connection between storage stability and the efficiency of vaccine or biologic drug merchandise is usually empirically evaluated, this discipline stands to learn as predictive stability fashions are higher tuned past standard approaches. Significantly within the context of the significance round future pandemic and world well being emergency readiness,6 stability modelling has advantages to supply shelf-life estimations for vaccines. Sometimes, such merchandise are saved at chilly temperatures, product course of/formulation adjustments are typically tougher when it comes to bridging, and the chemistry of the supplies concerned and related degradation pathways are sometimes complicated.
New method and benefits
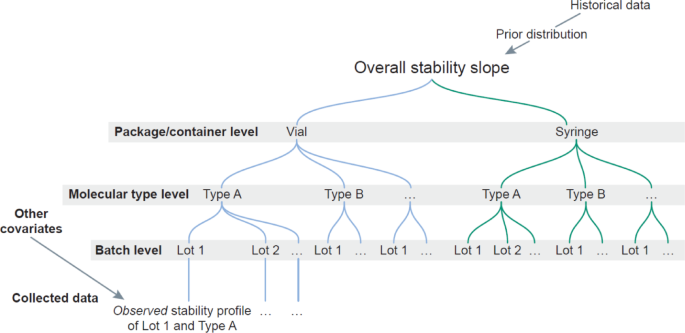
Given its capability to combine complicated multi-variate datasets,33 present coherent predictions, and generate credible interval estimates to quantify uncertainty, Bayesian statistical fashions have been efficiently employed for stability research. Extra particularly, hierarchical Bayesian fashions can incorporate a number of ranges of knowledge, corresponding to totally different batches, HPV sorts, or bundle/container, in a “tree” like construction to estimate parameters of pursuits and predict outcomes (Scheme 1). Because of this, Bayesian hierarchical fashions are capable of tackle complicated information buildings (for instance integrating details about totally different associated sub-groups) in addition to possessing an intrinsic mechanism for leveraging prior information.
As a result of inherent complexity of huge molecule formulations, in addition to co-formulation of a number of drug substances in some merchandise and multivalency widespread in vaccines, common acceptance and adoption of such predictive instruments was hardly ever adopted prior to now however have now change into an energetic consideration with world regulatory our bodies amidst an evolving panorama of trade pointers on stability monitoring and model-based prediction.
A number of technical, high quality, and regulatory limitations had traditionally existed for implementation of large-molecule predictive stability software for scientific and industrial merchandise. This paper goals to suggest and assist find out how to overcome many of those hurdles.
First, for late-stage R&D and industrial line of sight it’s important to supply true quantitative readouts of anticipated long-term stability and predicted shelf-life in relation to the soundness specs which might be set for the drug product on the idea of conventional stability research and statistical evaluation. Superior Bayesian hierarchical fashions present absolute quantitative predictions of stability in addition to extra information interpretation that may probably improve the management technique by deriving extra correct predictions from much less information, intuitive descriptions of credible intervals, better insights into covariate relationships, and making a singular unified mannequin framework to handle a number of varieties of questions. On this paper we current a completely quantitative Hierarchical Bayesian kinetic mannequin able to predicting stability and subsequent shelf-life in relation to drug product stability specs set for an HPV platform vaccine product.
Second, it’s fascinating to higher incorporate historic information in fashions, notably for well-established “legacy” industrial merchandise or round a molecular “franchise” composed of 1 drug substance shared and mixed throughout a number of drug product formulations and combos. For instance, traditionally a number of HPV vaccines have existed with increasing valency (evolution of 4-valent GARDASIL® sustaining the present antigen sorts together with addition of 5 new sorts into 9-valent GARDASIL®9). It might be extremely informative and speed up the trail to approval by higher incorporating historic stability information to match superior Bayesian fashions alongside the standard strategies of reporting stability to regulatory businesses. Right here we leverage historic information for greater than 30 batches of this platform vaccine product by way of the hierarchical modelling method.
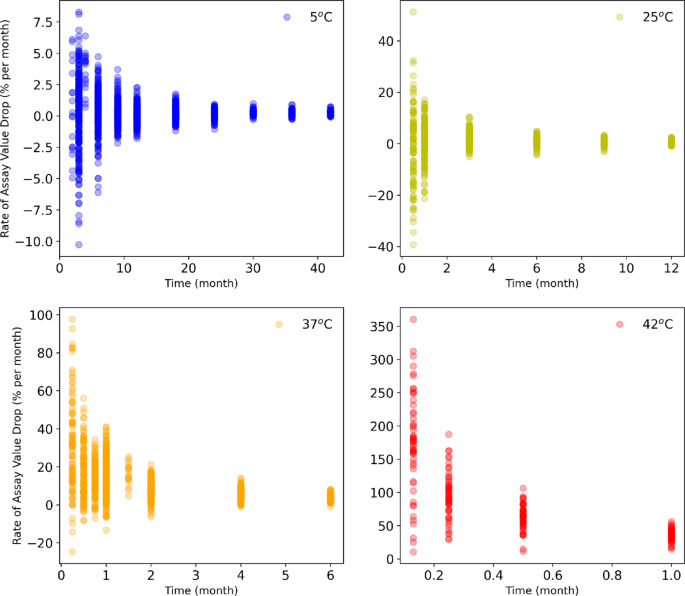
Third, it is necessary that the interpretability of stability fashions has a sturdy bodily foundation and adheres to logical biopharmaceutical/physicochemical understanding even when findings are empirical in nature. Subsequently, our fashions are of a hybrid kind that leverage statistical arithmetic along side an algorithm that mimics a biochemical response leveraging Arrhenius kinetics. Fourth, some large-molecule merchandise have vital random error in analytical assays resulting in excessive variability (“noisy information”), e.g., as is widespread for efficiency measurements. Our Bayesian mannequin addresses this variability adequately (as shall be additional mentioned). Lastly, one can’t assume that specialised tailor-made information units for the particular function of supporting predictive stability modelling will all the time be accessible in an industrial context, as a result of there are sometimes limitations in materials portions and confinements imposed by established customary working process (SOP) take a look at protocols.
Right here we suggest a mannequin designed to work with a standard set of ICH-guided temperature and time level circumstances (for instance, as traditionally performed for GARDASIL®9). All information units had been derived from customary industrial stability information and never explicitly designed to assist predictive stability. It is usually acknowledged by the authors that there could also be instances sooner or later the place extra components/circumstances could also be wanted to boost the robustness of those fashions. To summarize, this work represents a big development within the software of predictive stability for giant molecules, on this case research of vaccines.