Yield of Chitosan
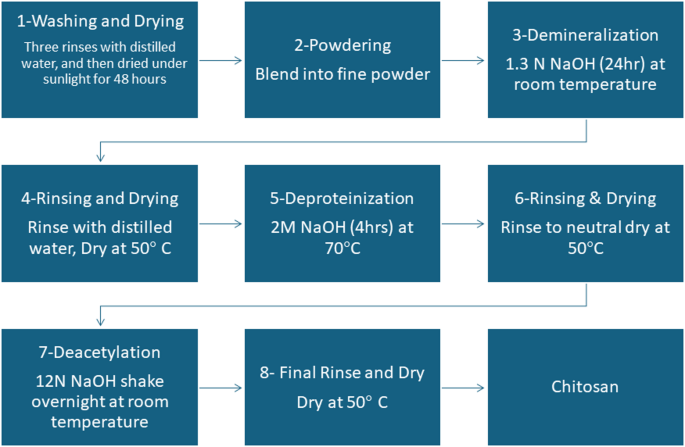
The yield of chitosan extracted from shrimps was (28 g / 120 g) × 100 = 23.33%. This outcome signifies that 23.33% of the unique shrimp shell waste was efficiently transformed into dry chitosan powder. The obtained yield falls inside the generally reported vary of 5.6–30% present in earlier research. As an example, research by Nanzin et al. reported chitosan yields between 7.5% and 18% utilizing comparable acid and alkaline concentrations, whereas different research by Nouri et al. 2016 achieved yields as excessive as 19.74 utilizing extra concentrated alkaline options. These outcomes recommended that the extraction methodology used was efficient and in step with established procedures32,33,34.
FT-IR spectroscopy
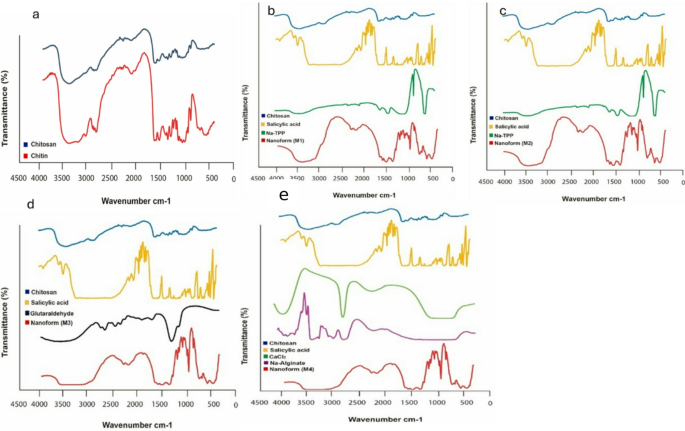
The FT-IR spectra of chitin and chitosan exhibited slight variations, primarily manifested as shifts of their bands (Fig. 3a). These shifts had been attributed to the lack of acetyl teams in chitosan. Chitin and chitosan exhibited absorption peaks at 3435 cm⁻¹ and 3443 cm⁻¹, respectively. These peaks corresponded to the stretching vibrations of -OH and amine N-H symmetric vibrations. Apparently, sharp absorption round 3500 cm− 1, indicative of free OH teams, was absent in all samples. The bands noticed at 1072 cm− 1 (chitin) and 1071 cm− 1 (chitosan) had been attributed to the stretching vibrations of -C-O teams, with chitosan exhibiting depressed C-O teams because of deacetylation. Moreover, the absorption band at 1413 cm− 1 in chitosan characterised the stretching vibration of the amino group. Additional attribute peaks between 1072 –1025 cm− 1 and 530–572 cm− 1 confirmed the saccharide construction of chitin and chitosan, respectively, owing to variations within the C-O teams35. Determine 3 (b and c) shows the FT-IR spectra of the primary and second types of chitosan-loaded salicylic acid nanoparticles, using TPP as a linker. A outstanding peak at 3450 cm− 1 corresponds to N-H and O-H stretching, in addition to intramolecular hydrogen bonds. This peak is notably sharper within the Cs-NPs, indicating enhanced hydrogen bonding36. The peaks noticed round 2923 cm− 1 and 2883 cm− 1 are attributed to C-H symmetric and uneven stretching, respectively. Moreover, the presence of residual N-acetyl teams is confirmed by peaks round 1629 cm− 1 (C = O stretching of amide I) and 1561 cm− 1 (NH2 teams). Apparently, these peaks shift to 1636 cm− 1 (M1), 1637 cm− 1 (M2), and 1555 cm− 1 (M1), 1556 cm− 1 (M2) within the FT-IR spectra of salicylic acid-chitosan-NPs, indicating interplay between NH3+ teams of chitosan and phosphate teams of TPP36,37. This interplay is additional supported by the lowered depth of the amide (1637 cm− 1) peak in salicylic acid-chitosan-NPs in comparison with pure chitosan. The presence of CH2 bending and CH3 symmetrical deformations is confirmed by bands noticed round 1410 cm− 1 and 1337 cm− 1, respectively38. Furthermore, the FT-IR spectrum of salicylic acid-chitosan-NPs displays attribute peaks of TPP at round 1214 cm− 1 (stretching vibration of P = O), 1144 cm− 1 (symmetric stretching vibrations within the O − P = O group), and at round 805 cm− 1 (uneven stretching vibration of the P − O−P bridge)38,39. Determine 3(d) exhibits the FT-IR spectrum of nano formulation 3 utilizing glutaraldehyde as a linker, illustrating delicate modifications within the surface-modified nanocarrier and nano-formulations. In salicylic acid-chitosan-NPs, distinct peaks had been noticed with shifts at 2316, 1552, 1051, and 641 cm-1. Moreover, new peaks appeared at 2236, 1943, and 471 cm− 1, which strongly signifies floor modification in comparison with the chitosan management. These spectral modifications are seemingly attributed to the presence of loaded salicylic acid inside the formulation. Determine 3(e) presents the FT-IR spectrum of nano formulation 4, illustrating overlapping peaks that counsel interactions amongst chitosan, sodium alginate, and salicylic acid. The spectrum reveals the presence of peaks from every part inside the nano formulation, indicative of their mixed presence and potential interactions. New peaks showing at 1961 and 1732 cm− 1 suggest chemical reactions involving salicylic acid with different components within the formulation. Key absorption bands embody a outstanding peak at 3396 cm− 1, attributed to the amine and hydroxyl teams current in each chitosan and alginate. The presence of a peak at 1067 cm− 1 signifies the presence of amino teams in chitosan. Moreover, the height at 1622.38 cm− 1 suggests an interplay involving the carboxylate group of chitosan with alginate interplay. These spectral options present useful insights into the complicated chemical interactions occurring inside nano formulation 4, highlighting the interaction amongst its elements40,41. M1 in Fig. 3b, exhibits completely different peak intensities in comparison with M2 in Fig. 3c, suggesting variations in crosslinking or molecular interactions. Sure peaks in Fig. 3c, shifted barely in comparison with Fig. 3b, indicating completely different levels of interplay between salicylic acid, Na-TPP, and chitosan. Further peaks in Fig. 3c: M2 displays further peaks and broadening within the amide (1600–1700 cm⁻¹) and phosphate areas (~ 1200 cm⁻¹), suggesting a stronger interplay or a unique structural association in comparison with M1 in Fig. 3b and M2 Fig. 3c displays better peak shifts, broadening, and extra built-in peaks, means that M2 is extra secure and uniform than M1. M1 Fig. 3b, has extra distinct and separate peaks, indicating weaker interactions and fewer stability.
Transmission Electron microscope TEM evaluation
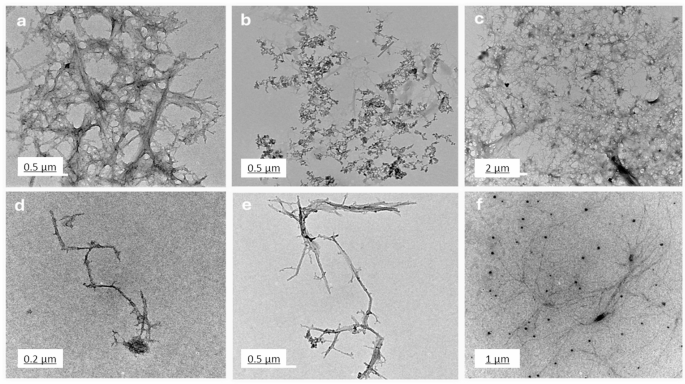
Determine 4 presents TEM pictures (a-f), highlighting the morphological variations amongst chitosan, salicylic acid, and salicylic acid-loaded chitosan NPs ready utilizing numerous strategies. Picture (Fig. 4a) illustrates a dense fibrous community of chitosan, seemingly shaped by intensive cross-linking. Figures (4b) depicts discrete salicylic acid particles with minimal aggregation, indicative of a preparation methodology that limits particle interplay. Pictures (Fig. 4c) by (Fig. 4f) symbolize salicylic acid-loaded chitosan NPs synthesized utilizing 4 distinct strategies. M1 (Fig. 4c), involving dissolvation /precipitation, exhibits clustered aggregates with irregular constructions, suggesting slower response charges or inadequate stabilization throughout synthesis42. M2 (Fig. 4d), using the TPP methodology, produces linear, chain-like nanoparticles ensuing from processes that promote linear polymerization or anisotropic development. M3, the glutaraldehyde methodology (Fig. 4e) generates branched, tree-like formations, achieved by situations favoring branched polymer development, comparable to various chitosan and cross-linker concentrations or facilitating self-assembly43 Lastly, the sodium alginate M4 (Fig. 4f) creates a dispersed distribution of nanoparticles, with efficient stabilization methods like ultrasonication or excessive shear mixing stopping aggregation. These TEM pictures emphasize the affect of preparation strategies on nanoparticle morphology, yielding various constructions comparable to dense networks, clustered aggregates, linear chains, and branched or dispersed formations. Understanding these variations is crucial for tailoring chitosan NPs for particular functions44. Primarily based on the TEM evaluation, the estimated dimension of the synthesized chitosan NPs various relying on the cross-linking methodology throughout the 4 strategies. The TPP-based methodology (M1 and M2) resulted in nanoparticles with a small and uniform dimension starting from 80 nm to 250 nm. Moreover, nanoparticles produced utilizing the glutaraldehyde methodology (M3) and sodium alginate methodology (M4) shaped bigger nanoparticles, starting from 150 nm to 400 nm, with network-like constructions. These outcomes confirmed that whereas TPP results in extra uniform, smaller particles, glutaraldehyde and sodium alginate produce bigger, community constructions.
The TEM pictures spotlight completely different chitosan nanoparticle morphologies and their potential functions. Community constructions (Picture a) are perfect for functions requiring excessive floor space and porosity, comparable to drug supply and tissue engineering. Clustered aggregates (Fig. 4c) could also be helpful in catalysis or adsorption processes45. Linear chains (Fig. 4d) are helpful for functions needing anisotropic properties, like nanocomposites or tissue scaffolds. Branched constructions (Fig. 4e) are advantageous for complicated architectures, comparable to superior drug supply methods. Dispersed particles (Fig. 4f) are optimum for functions requiring minimal aggregation, comparable to coatings or nanofluids. TEM evaluation (Fig. 4) revealed the formation of network-like nanostructures in our formulation. Whereas our research didn’t straight measure drug loading or launch kinetics, earlier analysis has proven that such nanostructures can improve drug loading capability and promote sustained launch46. Moreover, the presence of each networked and dispersed nanoparticles might counsel improved biofilm penetration. Tailoring synthesis strategies allows customization of nanoparticle properties for particular capabilities47.
EDX evaluation
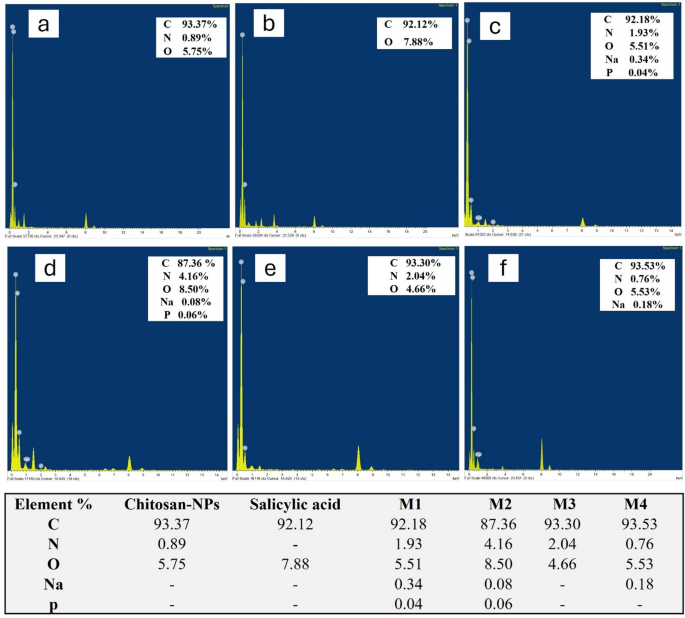
Determine 5 (a-f) presents the basic composition of varied substances as decided by EDX evaluation, offering perception into the chemical make-up of chitosan, salicylic acid, and 4 completely different nano formulations. The evaluation reveals that chitosan (Fig. 5a) is predominantly composed of carbon (93.37%), with oxygen making up 5.75% and a small quantity of nitrogen (0.89%). This elemental composition is in step with the molecular construction of chitosan, which primarily consists of carbon chains with oxygen and nitrogen purposeful teams48. In distinction, salicylic acid (Fig. 5b) displays a composition of 92.12% carbon and seven.88% oxygen, reflecting its natural, crystalline nature the place oxygen is a key part of the carboxyl and hydroxyl teams.
M1 (Fig. 5c), which incorporates chitosan NPs loaded with salicylic acid, exhibits a barely completely different elemental distribution. The carbon content material is 92.18%, oxygen is 5.51%, and nitrogen is 1.93%. Moreover, small quantities of sodium (0.34%) and phosphorus (0.04%) are current, seemingly as a result of incorporation of salts or crosslinking brokers through the nanoparticle preparation course of49. M2 (Fig. 5d), which additionally incorporates chitosan and salicylic acid, reveals 87.36% carbon, 8.50% oxygen, and a better nitrogen content material of 4.16%, indicating the presence of extra nitrogen-rich crosslinking brokers or components. Minor traces of sodium (0.08%) and phosphorus (0.06%) are additionally detected, suggesting that the formulation comprises different chemical elements that contribute to its structural properties50. For M3 (Fig. 5e), the basic composition exhibits 93.30% carbon, 4.66% oxygen, and a couple of.04% nitrogen. This formulation intently resembles the composition of chitosan, with a slight enhance in nitrogen, which can be as a result of introduction of further nitrogen-based crosslinking brokers or different stabilizers throughout synthesis. Lastly, M4 (Fig. 5f) displays a composition of 93.53% carbon, 5.53% oxygen, and 0.76% nitrogen, with small quantities of sodium (0.18%). The upper carbon and oxygen percentages counsel that the formulation is primarily composed of chitosan and its derivatives, with a minimal contribution from different components. The compositional variations noticed within the EDX evaluation affect the soundness, bioactivity, and interactions of the nanoparticles with microbial cells. The variations in nitrogen content material throughout formulations counsel variations in crosslinking density, which might affect nanoparticle stability. Increased nitrogen ranges in M2 and M3 point out the presence of nitrogen-rich crosslinkers, which improve structural integrity and affect drug launch kinetics51. The presence of sodium in M1, M2, and M4 suggests potential ionic interactions that may have an effect on nanoparticle dispersion, stopping agglomeration and floor cost, influencing stability in organic environments52. Moreover, phosphorus detected in M1 and M2 might point out residual crosslinking brokers or stabilizers that might alter nanoparticle floor properties, affecting microbial adhesion and interplay. Oxygen content material variations counsel variations in hydrophilicity, which might affect solubility, bioavailability, and penetration into microbial biofilms53. M2, with the best oxygen content material, might exhibit enhanced water affinity, probably enhancing drug launch and biofilm penetration. Conversely, formulations with increased carbon content material (M3 and M4) counsel a extra hydrophobic nature, which might affect mobile uptake and antimicrobial exercise54. Total, the EDX evaluation gives an in depth understanding of the basic make-up of the assorted formulations, indicating the profitable incorporation of chitosan and different elements like salicylic acid, crosslinking brokers, and salts. The variations in nitrogen, sodium, and phosphorus content material throughout the formulations spotlight the affect of various synthesis strategies and crosslinking brokers on the ultimate chemical composition, which performs a vital function in figuring out the nanoparticles’ properties and their potential functions55,56.
Morphology of Chitosan NPs (SEM)
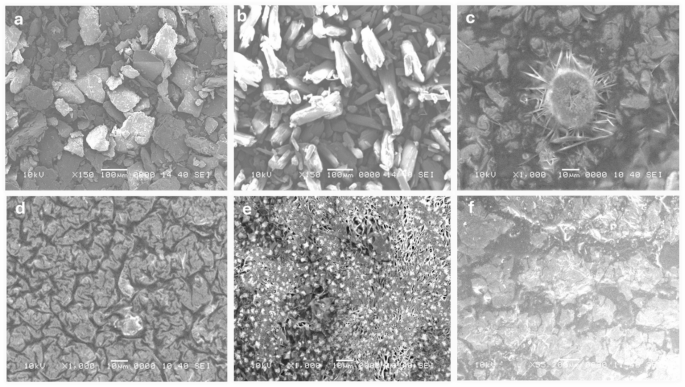
The SEM pictures (Fig. 6 (a-f)) reveal the morphological variations in chitosan and its composites ready by numerous strategies. Bulk chitosan (Fig. 6a) exhibits an irregular, flaky floor typical for biopolymers of their unmodified type57. Salicylic acid (Fig. 6b) displays crystalline constructions with sharp edges, reflecting its small molecule, crystalline nature. Chitosan NPs ready by desolvation (Fig. 6c) show a singular star-shaped morphology with needle-like projections. TPP-crosslinked chitosan NPs (Fig. 6d) have a wrinkled floor and a extra uniform, densely packed look. Glutaraldehyde-prepared nanoparticles (Fig. 6e) present a porous construction, indicating profitable crosslinking. Lastly, sodium alginate-prepared nanoparticles (Fig. 6f) are tough and agglomerated, suggesting interactions between chitosan and alginate. Star-shaped nanoparticles (desolvation methodology) might improve bacterial membrane disruption because of their excessive floor space and sharp edges, whereas wrinkled, densely packed nanoparticles (TPP-crosslinked) supply better stability and sustained drug launch, supporting extended antibiofilm motion. Porous nanoparticles (glutaraldehyde-crosslinked) permit for increased drug encapsulation and managed diffusion, enhancing bacterial eradication, whereas tough, agglomerated nanoparticles (sodium alginate-crosslinked) might have an effect on dispersion and biofilm penetration, probably influencing antimicrobial effectivity. These morphological variations spotlight the affect of various preparation strategies and crosslinking brokers on nanoparticle formation58,59,60.
The variation in particle dimension, form, and floor morphology exhibits that the selection of crosslinking agent and preparation methodology considerably impacts chitosan NPs’ properties. TPP crosslinking presents uniformity and density, whereas glutaraldehyde creates porosity, helpful for drug supply. These findings underscore chitosan’s versatility and the significance of choosing the correct strategies to tailor its properties for particular functions. Future research might give attention to drug loading, launch kinetics, and biocompatibility to discover their biomedical potential61,62.
Particle dimension distribution
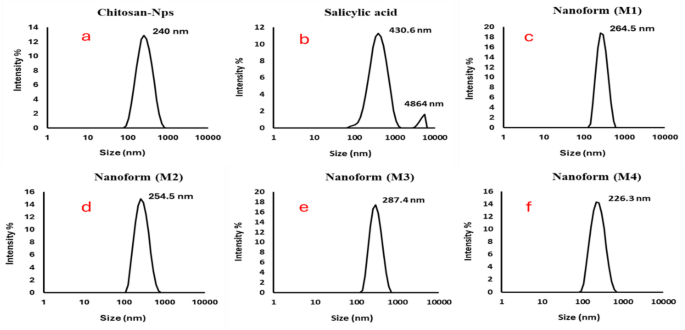
Determine 7 (a-f) exhibits a complete evaluation of the scale distribution and zeta potential of chitosan, salicylic acid, and salicylic acid-loaded chitosan NPs ready utilizing numerous strategies. The DLS evaluation reveals a slender dimension distribution for all of the nanoparticle formulations, with mono dispersity indices of 0.139 for chitosan (Fig. 7a), 0.261 for salicylic acid (Fig. 7b), and 0.118, 0.135, and 0.166 for the completely different salicylic acid-loaded chitosan nanoparticle preparations (M1 (Fig. 7c), M2 (Fig. 7d), M3(Fig. 7e), and M4 (Fig. 7f), respectively). These indices point out comparatively uniform particle sizes, with a mean diameter of roughly 254 nm throughout all samples. The consistency of the particle sizes measured by DLS aligns intently with the particle sizes noticed within the TEM pictures, offering further affirmation of the accuracy of the nanoparticle measurements63.
Moreover, Desk 1 gives the zeta potential measurements that provide essential insights into the soundness and dispersibility of the nanoparticles. Chitosan NPs exhibit a zeta potential worth of 47.6 mV, whereas salicylic acid alone exhibits a a lot decrease worth of -2.3 mV, reflecting its much less secure nature. The salicylic acid-loaded chitosan NPs, ready by the 4 completely different strategies, present zeta potential values starting from + 36.6 mV to 41.3 mV for strategies 1 to 4, respectively. These optimistic zeta potential values counsel that the nanoparticles are well-dispersed, with robust electrostatic pressure between particles, stopping aggregation and guaranteeing their stability in suspension. The upper optimistic zeta potential values noticed for the salicylic acid-loaded chitosan NPs point out enhanced dispersion and stability, which is helpful for his or her potential use in numerous functions, together with drug supply methods and biomedical formulations64. The info introduced in Fig. 4; Desk 1 not solely spotlight the uniformity in dimension distribution but in addition emphasize the significance of the preparation methodology in controlling the nanoparticle traits, comparable to particle dimension, dispersion, and stability55,58. These properties are important for tailoring the nanoparticles for particular functions, and the outcomes counsel that cautious number of preparation methods can optimize the purposeful properties of chitosan NPs for focused use in fields comparable to drug supply, tissue engineering, and nanomedicine60,63.
Cell viability assay
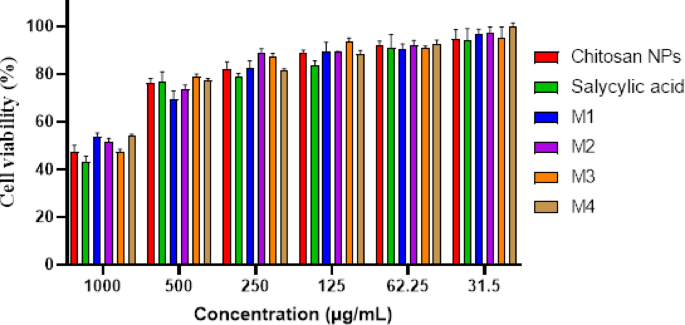
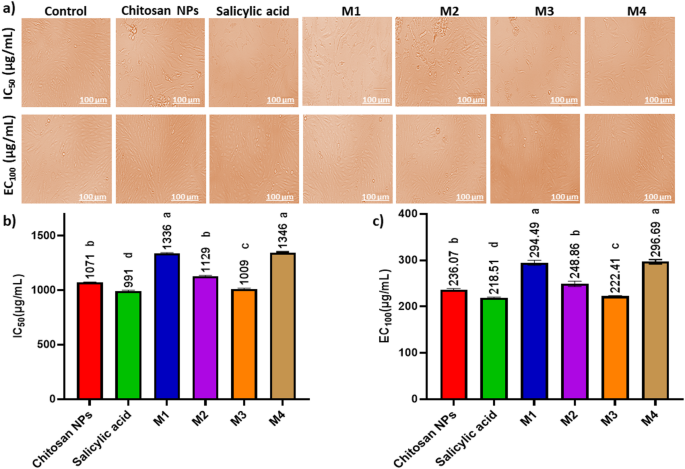
Determine 8; Desk 2 signifies a dose-dependent cytotoxic impact of the examined formulations on HSF cells, the place increased concentrations (1000 µg/mL) resulted in decrease cell viability, whereas decrease concentrations (≤ 31.5 µg/mL) confirmed minimal cytotoxicity. Determine 9a exhibits the morphological viability of HSF after therapy with the examined compounds. Determine 9b, c) illustrate the 100% protected focus vary, which spans from 218.51 to 296.69 µg/mL, with salicylic acid recognized because the least protected compound. In response to ISO 10993-5 biocompatibility pointers, a fabric is taken into account non-cytotoxic if cell viability stays ≥ 70% in comparison with the detrimental management. This reinforces that our nanoformulation is appropriate for biomedical functions, together with wound therapeutic and antibiofilm coatings, whereas sustaining each security and efficacy. Moreover, the pure origin, biodegradability, and FDA-approved standing of chitosan additional assist its medical applicability65. Nonetheless, the nanoforms ready utilizing strategies 1 and 4 (M1 and M4) demonstrated the best EC100 values, making them the most secure nanoparticles. The IC50 values of chitosan NPs fall inside the revealed vary of 20 to 2500 µg/mL66,67. Moreover, the IC50 of the salicylic acid on this research was 991 µg/mL aligning with the reported IC50 at 828.72 µg/mL68.
Impact of the protected dose (EC100) and 50% inhibitory concentrations (IC50) of the examined compounds on human pores and skin fibroblast cells (HSF). (a) Morphological modifications captured utilizing a phase-contrast microscope. (b) IC50 values (the focus at which 50% inhibition happens) (µg/mL) of the examined compounds (c) EC100 values (the focus at which 100% impact is noticed) (µg/mL) of the examined compounds. Knowledge are introduced as imply ± customary deviation (SD). Totally different superscript letters point out important variations throughout rows (p
Antibiofilm Inhibition assay
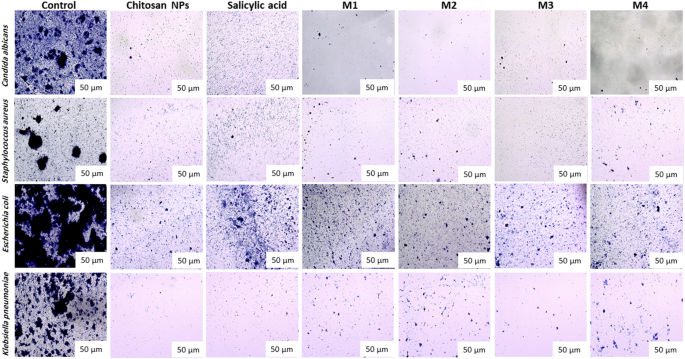
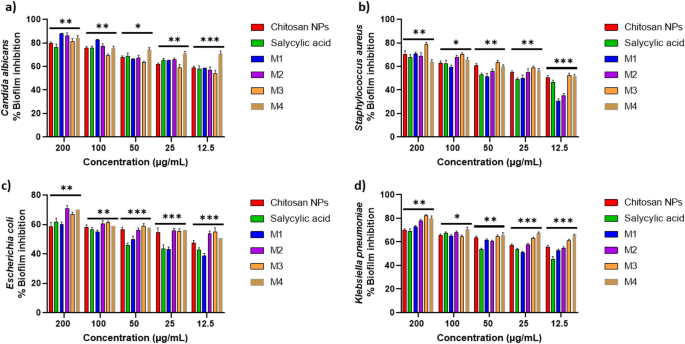
The compounds exhibit a big antibiofilm impact in opposition to the examined microorganisms at their protected concentrations. Determine 10 presents the qualitative morphological observations beneath a lightweight microscope, illustrating the power of the examined compounds to take away pre-formed biofilm after 24 h. Determine 11(a–d) presents quantitative information on the proportion inhibition of biofilm formation by the examined microorganisms: (a) Candida albicans, (b) Staphylococcus aureus, (c) Escherichia coli, and (d) Klebsiella pneumoniae. The nano-formulations demonstrated a better proportion of inhibition, probably because of their better floor negativity. The nanoform combining chitosan and salicylic acid exhibits superior efficacy in comparison with both compound alone in opposition to nearly all examined microorganisms. The % inhibition of chitosan NPs and different nanoforms is dose-dependent and proportional to the focus69,70.
Some research have constantly attributed chitosan’s distinctive antibiofilm properties to its polycationic nature, which arises from the purposeful amino teams (NH₂) current within the N-acetylglucosamine models. Moreover, the polymeric construction of chitosan and chitosan oligosaccharides allows their chelation with important metals comparable to calcium, zinc, and magnesium. These metals play essential roles in bacterial gene transcription and translation; their sequestration disrupts these processes, finally resulting in cell dying71. Moreover, chitosan NPs exhibit anti-quorum sensing (anti-QS) exercise by interfering with bacterial cell-to-cell communication mechanisms or inhibiting the quorum sensing signalling pathways. This disruption prevents the formation of molecule-receptor complexes and the synthesis of varied signalling molecules, successfully hindering biofilm improvement72. Moreover, chitosan has been utilized to safeguard medical gadgets, comparable to catheters and orthopedic implants, in opposition to infectious biofilm-forming pathogens. Chitosan coatings have additionally demonstrated the power to eradicate substantial quantities of pre-existing viable biofilms of Staphylococcus epidermidis and Staphylococcus aureus on implant surfaces. This lively protecting impact of chitosan coatings on medical system surfaces represents a important development in decreasing the excessive danger of device-associated infections71,73.
Biofilm inhibition proportion of various concentrations (200 –12.5 µg/mL) of the examined compounds in opposition to (a) Candida albicans; (b) Staphylococcus aureus; (c) Escherichia coli; (d) and Klebsiella pneumoniae. The bars on the graphs symbolize the imply ± SD of three impartial replicates. The numerous variations between teams: *p p p
Desk 3 presents the fold discount in biofilm formation. M1 achieved superior inhibition of Candida albicans biofilms (8.31-fold discount), surpassing M2 (7.22-fold discount). This efficiency aligns with findings by Gondim et al.69, emphasizing the function of polycationic nature in antibiofilm exercise. M3 exhibits important discount in opposition to each Staphylococcus aureus and Klebsiella pneumoniae. Among the many examined microorganisms, Escherichia coli displays the least fold discount in biofilm formation, with M2 being the best formulation. Positively charged nanoparticles are simpler antibacterial brokers because of their robust electrostatic attraction to negatively charged bacterial membranes, different elements comparable to form and dimension additionally considerably affect their antibacterial efficacy74. Positively charged NPs improve bacterial adhesion, disrupt cell constructions by ion change, and promote reactive oxygen species (ROS) manufacturing, making them extremely efficient75.
The notable antibiofilm impact in opposition to Candida albicans (85% inhibition) could be attributed to chitosan’s skill to compromise fungal cell membranes, induce cytoplasmic leakage, sequester important vitamins, and disrupt genetic processes. These mechanisms are influenced by elements comparable to molecular weight, diploma of deacetylation, and the composition of the fungal cell membrane, notably its unsaturated fatty acid content material, which performs a task in figuring out susceptibility. Chitosan-based nanoparticles additional amplify these results because of their better floor cost, expanded floor space, and enhanced mobile uptake, facilitating deeper biofilm infiltration and superior antifungal exercise in comparison with typical chitosan12. Whereas chitosan’s antimicrobial efficacy varies amongst completely different microbial species—with some research indicating increased effectiveness in opposition to Gram-negative micro organism76 and others reporting better sensitivity in Gram-positive strains77—its robust inhibitory affect on Candida albicans on this research underscores its potential as a promising antifungal and antibiofilm agent.