Cells and viruses
Expi293 cells have been used for protein expression. Monkey kidney epithelial Vero cells have been used for virus neutralization assays. Soluble E protein sequences have been primarily based on DENV1 WestPac 74 (aa 1-394), DENV2 16681 (aa 1-394), DENV3 CH53489 (aa 1-392) and DENV4 TVP-376 (aa 1-394). Mature viruses produced in Vero cells overexpressing furin protease (VeroFurin cells) have been used for binding and neutralization experiments50,51.
Protein expression and purification
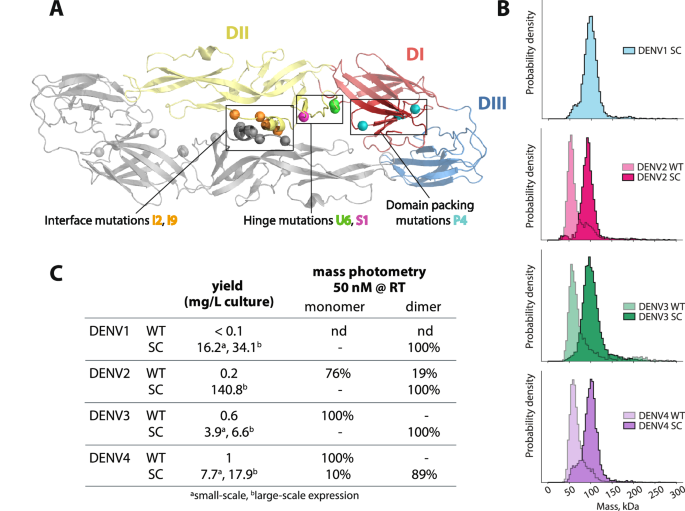
DNA plasmids have been amplified from DH5α cultures and purified utilizing endotoxin-free DNA extraction kits (Macherey-Nagel) as beforehand described in ref. 28. Purified plasmids have been then transfected into Expi293 cells utilizing Expifectamine reagent in response to the producer’s tips for small scale (25–50 mL) or giant scale (200–500 mL) manufacturing. Cells have been grown in a 37 °C, 8% CO2 incubator at 130 rpm shaking. Media containing secreted proteins have been harvested 64–72 h publish transfection, and clarified by centrifugation at 3500 g (small scale expression) of 10,000 g (giant scale expression) for 15 min. The supernatant was filtered by means of a 0.2 µm membrane and saved at 4 °C till purification.
Purification of sE was completed utilizing Nickel affinity column adopted by gel filtration. Briefly, Nickel Penta resin (Marvelgent) was equilibrated with 10 column quantity (CV) PBS and incubated with protein media in batch binding mode for 1 h or in a single day at 4 °C. Resin was washed with 6 CV (1 M tris-HCl, 0.5 M NaCl, 25 mM imidazole, pH 8.0), 1 CV PBS + 25 mM imidazole pH 7.4 and 1 CV PBS + 50 mM pH 7.4. Protein was eluted with 1x PBS + 500 mM imidazole pH 7.4, concentrated and buffer exchanged into PBS + 10% glycerol pH 7.4 buffer for storage.
Liposome synthesis
Supplies
The next lipids have been used to type CoPoP/PHAD/QS21 liposomes (CPQ); 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DOPC, Corden # LP-R4-070), ldl cholesterol (PhytoChol, Wilshire Applied sciences), artificial monophosphoryl Hexa-acyl Lipid A, 3-Deacyl (PHAD-3D6A, Avanti Cat # 699 855), and QS-21 (Desert King). Cobalt-porphyrin-phospholipid (CoPoP) or porphyrin-phospholipid (PoP).
Liposome preparation
CPQ liposomes have been ready by an ethanol injection methodology, adopted by nitrogen-pressurized lipid extrusion in a phosphate-buffer saline (PBS) carried out at 55 °C. Lipids have been dissolved in 1 mL pre-heated 55 °C ethanol for 10 min, then 4 mL of pre-heated PBS have been added and incubated at 55 °C for 10 min. The liposome extruder (Northern Lipids) was nitrogen pressurized and heated to 55 °C with a stress of close to 200–300 PSI. Then the liposomes have been extruded by means of 200,100 and 80 nm membrane filters stack for 10 instances, adopted by dialyzing in PBS at 4 °C twice to take away ethanol. Later, liposomes have been handed by means of a 0.2 μm sterile filter, and QS-21 (1 mg/mL) have been admixed with liposomes, the ultimate focus was adjusted to 320 µg/mL of CoPoP, 128 µg/mL of PHAD and 128 µg/mL of QS21 and saved at 4 °C. Illustrations within the manuscript have been generated with Adobe Illustrator (model 29). Protein photos have been generated with PyMol (model 1.20).
LPQ and CPQ liposome formulation
sE proteins have been conjugated on CPQ liposomes following beforehand described protocols34,35. Particularly, the proteins have been incubated with CPQ at a mass ratio 1:4 sE (monomer):CoPoP for 4 h at room temperature at the hours of darkness. LPQ liposomes have been additionally incubated with proteins in the identical method utilizing 1:4 sE (monomer):PoP mass ratio. After the incubation, the samples have been shielded from gentle and saved at 4 C.
CPQ-sE conjugation effectivity evaluation by Ni binding and SDS-PAGE
25 µL sE or liposomes incubated with sE have been incubated with both 5 µL 50% Ni resin slurry preequilibrated in PBS or PBS for 30 min at room temperature at the hours of darkness. The ultimate protein concentrations have been 40 µg/mL. The samples have been blended gently by pipetting each 10 min, after which transferred to a spin column and centrifuged for 1 min at 2000 g to separate the Ni beads and the answer. Ni beads have been resuspended utilizing 27.5 µL PBS into a brand new tube. Flowthrough fractions and Ni beads have been analyzed by decreasing SDS-PAGE to evaluate for the presence of unconjugated sE in resolution.
Dimension evaluation of LPQ and CPQ liposomes pre- and post- sE conjugation by dynamic gentle scattering (DLS)
DLS measurements have been carried out on a DynaPro Plate Reader II instrument in isothermal mode. Liposome samples have been diluted in PBS to a ultimate focus of 100 µg/mL (Co)PoP. 2 mg/mL BSA in PBS was used as a management. The typical hydrodynamic radius and polydispersity from 5 acquisitions (5 s every) was plotted.
Antibody binding to DENV1-4 sE and CPQ-sE by enzyme-linked immunosorbent assay (ELISA)
Binding research have been completed in response to beforehand printed protocols28. Soluble E proteins with a C-terminal His-tag (50 µL at 2 ng/µL) have been straight coated on Nickel plates (Pierce, cat# 15142) in TBS buffer for 1 h at 37 °C. Plates have been washed thrice with 0.2% TBS Tween-20 (200 µL/nicely). Human anti-E monoclonal Abs have been added to the plates at 2 ng/µL in blocking buffer (1x TBS buffer pH 7.4 containing 0.05% v/v Tween 20 and three% non-fat dry milk) and incubated for 1 h at 37 °C, 300 rpm (50 µL/nicely). Plates have been washed then incubated with goat anti-human IgG conjugated with alkaline phosphatase (AP) for 45 min at 37 °C. After a ultimate wash, wells have been developed with pNPP. Binding was correlated with absorbance at 405 nm. The assays have been carried out in duplicate.
To seize liposome formulated sE proteins (CPQ-sE), human anti-E antibody 1M7 was coated in a 96-well plate (Greiner, cat# 655061) in a single day at 4 °C at 100 ng/nicely in 0.1 M NaHCO3 pH 9.6. Wells have been blocked 1 h at 37 °C. CPQ-sE samples have been diluted to 2 ng/µL sE in blocking buffer and added to the plate for 1 h at 37 °C, 300 rpm (50 µL/nicely). AP-conjugated human anti-E mAbs (utilizing Abcam equipment, cat# ap102850) have been probed and detected by pNPP as described above. Further binding research have been completed with mouse mAb 4G2 because the seize Ab. For 4G2-captured samples, we probed binding with unlabeled human mAbs and goat anti-human IgG-AP (0.5 ng/µL).
Mouse immunogenicity research
Feminine BALB/c mice have been bought from Jackson Laboratory and used at 9–10 weeks of age. For every immunization, mice got an intramuscular injection (beneath no anesthesia) of 30 μL vaccine formulations in PBS within the thigh muscle tissue of every hind limb, a complete of 60 μL per mouse. For the monovalent formulations of DENV2 and DENV3 vaccines, every mouse obtained 2 μg sE (WT or steady dimer) formulated with LPQ or CPQ liposomes (n = 6). Bivalent formulations blended 2 μg sE of DENV2 or DENV3 with the liposomes, after which blended the liposomes (n = 6). For the tetravalent formulation,1 μg of every sE was blended with CPQ liposomes, after which the liposomes have been mixed. Management teams included 2 μg DENV2 and a couple of μg DENV3 sE+125 μg Alum (n = 6), CPQ or LPQ alone (n = 3). All teams have been immunized with the identical antigen formulation and dose at day 0 and 28, and serum samples have been collected at indicated time factors. Mice have been euthanized on the finish of research by way of CO2 inhalation (7 liter/minute) until respiration ceases, adopted by cervical dislocation.
Ethics assertion
All experiments involving mice have been carried out in response to the animal use protocol authorized by the College of North Carolina Animal Care and Use Committee. The animal care and use associated to this work complied with federal rules: the Public Well being Service Coverage on Humane Care and Use of Laboratory Animals, Animal Welfare Act, and adopted the Information for the Care and Use of Laboratory Animals.
Mouse serum binding ELISA to complete virus
The protocol is much like the seize ELISA described above. Human 1M7 antibody was immobilized on Greiner plate (cat # 655061) in 0.1 M NaHCO3 buffer pH 9.6 (100 ng/nicely) in a single day at 4 °C. All incubations on the next day have been completed at 37 °C with light shaking. Plates have been blocked the subsequent day with blocking buffer for 1 h. Mature viruses cultured in Vero Furin cells have been captured on the plate for 1 h. Following washes, mouse serum was serially diluted in blocking buffer and added to the virus-captured plate (50 µL/nicely) and incubated for 1 h. Plates have been washed, and anti-mouse IgG-AP (Sigma-Aldrich) was added to the wells (50 µL/nicely) for a forty five min incubation. After a ultimate wash, sign was developed utilizing pNPP, monitoring for absorbance at 405 nm. Assays have been carried out in duplicates. The binding IgG titer was calculated because the midpoint dilution (Fig. 3) and space beneath the curve (Supplementary Fig. 4) of placebo-subtracted binding sign in opposition to serum dilutions. We tried to depict this measurement as antibody focus by comparability to the monoclonal antibody 4G2. Nevertheless, as 4G2 binds differentially to DV1-4 (Supplementary Fig. 7), the focus approximation shouldn’t be consultant of the true polyclonal sera. Full binding curves can be found in Supplementary Figs. 8–11. All graphs within the manuscript have been generated with GraphPad prism (model 10.1.2).
Focus discount neutralization check (FRNT) by mouse sera
Vero cells have been seeded the evening earlier than at a density of 20,000 cells/nicely and needs to be at 80–90% confluency on the day of the experiment. Vaccinated mouse serum was serially diluted in development medium (1% Antibiotic-Antimycotic (Gibco), 1% MEM Non-Important Amino Acids Resolution (Gibco), 1% L-Glutamine (Corning) in DMEM F12 (Gibco) containing 2% heat-inactivated FBS and incubated with virus for 1 h at 37 °C. The virus-serum combination was then added onto cells (30 µL/nicely). After an hour incubation at 37 °C, the combination was faraway from cells and overlay media (OptiMEM (Gibco) + 2% HI FBS + 1% Anti-anti + 1% (w/v) carboxymethylcellulose (Sigma-Aldrich)) was added to the plate (180 µL/nicely). Foci have been allowed to develop for a various period of time. The incubation time for mature DENV1 is 48 h, DENV2 is 52 h, DENV3 is 52 h and DENV4 is 44 h. After the an infection incubation window, overlay media was flicked off from the wells. Cells have been washed with PBS and stuck with 4% PFA for 30 min at room temperature, and subsequently washed with PBS.
To visualise foci on mounted cells, cells have been permeabilized with 1x PERM ((10X PERM buffer was diluted in MilliQ water. 10X PERM: 1% of bovine serum albumin (BSA, Sigma)) for 10 min at room temperature (50 µL/nicely) or in a single day (100 µL/nicely). 1 ng/µL 1M7 antibody in PERM + 5% milk was added to every nicely for 1 h at 37 °C. Cells have been washed with 1x PBS and incubated with 1:4000 anti-human IgG-HRP (Southern Biotech) for 1 h at 37 °C. After washing with PBS, 30 µL KPL True blue peroxidase substrate (SeraCare) was added to every nicely. After 15–30 min growth at room temperature, wells have been rinsed beneath a mild stream of water and let dry. Wells have been imaged utilizing Immunospot digital camera and foci have been counted utilizing Viridot software program53. Focus discount was calculated as: % neutralization = (fociplacebo – fociserum)/fociplacebo*100%. Knowledge plotted as common of duplicates and fitted to a sigmoidal dose-response curve. Full virus neutralization curves can be found in Supplementary Figs. 8–11.
Depletion of sE-specific Ab from pooled polyclonal sera
sE proteins coupled to HisPur magnetic beads (ThermoFisher, cat# 88832) have been used to tug out corresponding binding Abs. Beads have been washed and equilibrated in PBS 0.05% Tween-20 containing 20 mM imidazole. Proteins have been incubated with beads at 0.08:1 mass ratio in the identical buffer. The ultimate coupled beads have been washed and saved in PBS pH 7.4.
To deplete anti-sE Abs from polyclonal serum, we used 32 µg proteins coupled to beads (400 µg beads) for every spherical of depletion. The beads have been added to 96-well plate and washed with 100 µL PBS. A magnetic plate was used to pellet the beads and the plate was flicked to take away the answer. 170 µL 10-fold diluted pooled sera in PBS + 5% BSA was added to the corresponding depletion arrange and beads have been resuspended gently by means of pipetting. Any empty wells have been stuffed with 150 µL PBS to reduce pattern loss attributable to evaporation and the plate was rigorously sealed. Sera and proteins have been incubated for 1 h at 37 °C at 650–800 rpm in a thermomixer (Eppendorf) to make sure correct agitation. As soon as accomplished, the plate was spun at 4000 rpm for five min and a magnetic plate was used to separate the beads from the sera. Sera was rigorously aspirated from the wells to keep away from mixing with beads. A adverse management for depletion was carried out by coating beads with BSA on the identical mass ratio talked about above. Every serum pattern was depleted 3 instances with the antigens listed in Fig. 4B. Depletions have been validated utilizing Ni ELISA checking for binding of polyclonal serum with the depleting antigen (e.g. if serum was depleted with DENV2 sE SC, depletion is full if there is no such thing as a longer binding of serum to DENV2 sE SC in Ni ELISA, Supplementary Fig. 6). After depletions, sera have been assessed for lack of neutralization at dilution issue 420 by normalizing the foci rely to the management BSA-depleted sera. Experiments have been carried out in duplicates.




