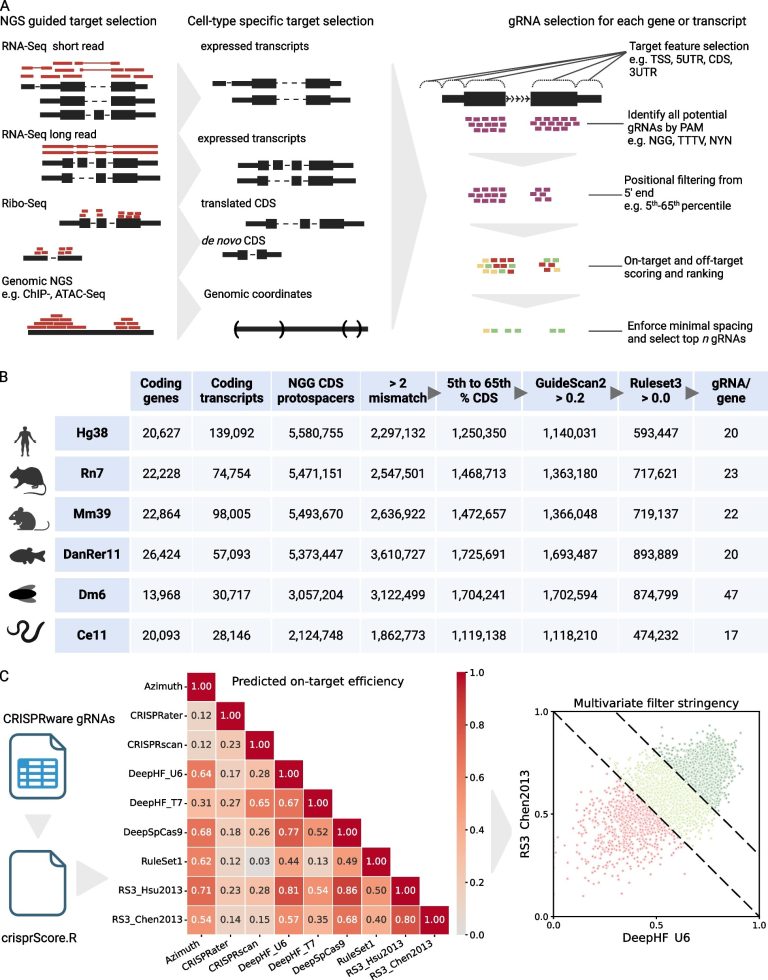
In a groundbreaking development poised to revolutionize the way forward for crop genetic engineering, scientists have unveiled a novel technique for delivering CRISPR–Cas9 genome-editing instruments into wheat vegetation utilizing an RNA virus-based vector. This technological breakthrough addresses a significant bottleneck in plant biotechnology — the environment friendly and heritable supply of gene-editing elements into monocot species, which embody necessary cereal crops comparable to wheat, rice, and maize. The examine leverages the distinctive biology of barley yellow striate mosaic virus (BYSMV), remodeling it into a sturdy supply system able to reaching the rising factors of wheat axillary meristems and producing secure, heritable genome modifications with out the necessity for transgenic constructs or tissue tradition procedures.
Standard approaches to plant genome modifying face quite a few challenges, particularly in monocots, the place transformation methods are sometimes inefficient, genotype-dependent, and labor-intensive. Conventional strategies sometimes require the insertion of overseas DNA by means of tissue tradition regeneration, which may induce somaclonal variations and delay breeding timelines. The newly engineered BYSMV vector circumvents these limitations by using a negative-strand RNA virus platform, which naturally infects monocot species and propagates systematically inside host vegetation. By repurposing the virus as a gene supply car, the analysis group succeeded in introducing each the Cas9 endonuclease and the particular single information RNAs (sgRNAs) into wheat cells in a tissue culture- and transgene-free method.
On the coronary heart of this revolutionary system lies a complicated molecular technique that dramatically enhances the mobility and concentrating on of genome-editing reagents. The researchers found that fusing a cell switch RNA (tRNA) sequence to the mRNA encoding Cas9, in addition to to the sgRNAs, permits environment friendly transport of those molecules into the axillary meristems — the vital development areas answerable for producing tillers, or aspect shoots, in wheat vegetation. This ingenious fusion trick ensures that the gene-editing elements arrive exactly the place heritable adjustments can happen previous to tiller technology, a step essential for passing mutations on to subsequent progeny.
.adsslot_7zqWa3Yrls{ width:728px !necessary; top:90px !necessary; }
@media (max-width:1199px) { .adsslot_7zqWa3Yrls{ width:468px !necessary; top:60px !necessary; } }
@media (max-width:767px) { .adsslot_7zqWa3Yrls{ width:320px !necessary; top:50px !necessary; } }
ADVERTISEMENT
The power to edit a number of homoeoalleles concurrently was one other exceptional side of this technique. Wheat is a hexaploid species containing three distinct genomes, every harboring homologous gene copies referred to as homoeoalleles. Concentrating on all three alleles in a single go is notoriously tough attributable to genetic redundancy and sequence similarities. Nevertheless, the BYSMV-based supply system achieved simultaneous mutations throughout all focused homoeoalleles, thereby producing nascent tillers bearing compound edits — a feat that considerably accelerates useful genomics evaluation in addition to breeding packages aiming to introduce advanced traits.
Importantly, the progeny vegetation derived from contaminated dad and mom have been confirmed to be virus-free, eliminating issues about viral persistence or off-target results stemming from extended virus an infection. Furthermore, the mutations recognized in these seedlings have been both bi-allelic or homozygous, signifying secure inheritance of edits and the potential for quick use in breeding efforts with out the need for additional backcrossing or choice. This end result displays the excessive precision and reliability of the viral supply platform, which might signify a transformative step towards non-transgenic crop enchancment.
Using BYSMV as a gene supply vector is especially strategic given its pure host vary, which spans a formidable 26 monocot species. This broad infectivity hints on the versatility of the system past wheat alone, opening avenues for genome modifying throughout a various array of cereal crops and grasses. Such scalability is essential in fashionable agriculture, the place fast adaptation to local weather change, pest strain, and evolving client calls for require swift and versatile breeding options.
Whereas earlier efforts in virus-mediated supply have sometimes concerned positive-strand RNA viruses, the present examine illustrates the efficient engineering of a negative-strand RNA virus spine, increasing the viral toolbox out there for plant biotechnology. Detrimental-strand RNA viruses current distinctive advantages together with their replication mechanisms and genomic architectures, which might be harnessed for secure expression and correct supply of genome modifying elements. This methodological innovation broadens the horizons of plant genome engineering by offering various viral vectors with numerous properties suited to various crop species and experimental aims.
The combination of a cell tRNA fusion sequence into the Cas9 and sgRNA transcripts harnesses the plant’s intrinsic intercellular trafficking mechanisms, a sublime exploitation of host biology to boost supply effectivity. By co-opting the endogenous pathways for RNA mobility, the expertise ensures that modifying equipment not solely reaches proliferating cells but additionally persists lengthy sufficient to enact heritable genome modifications. Such deep understanding of molecular plant biology mixed with viral vector engineering exemplifies the delicate, interdisciplinary method required to push the boundaries of crop genome modifying.
This RNA virus-based supply system additionally addresses main regulatory and public acceptance hurdles related to transgenic and tissue culture-based genetic modifications. For the reason that technique leads to virus-free progeny devoid of overseas DNA integration, the edited vegetation are successfully non-transgenic, thereby simplifying regulatory pathways and doubtlessly lowering public resistance to genome-edited crops. By enabling non-transgenic, heritable genome modifying that bypasses classical genetic engineering bottlenecks, the BYSMV vector system emerges as a strong device for next-generation crop breeding.
Past elementary analysis, the implications for international meals safety and sustainable agriculture are profound. Wheat is a staple crop feeding billions worldwide, and improvements that speed up its genetic enchancment can have ripple results all through the agri-food sector. By facilitating fast and exact modifying of essential agronomic traits — together with yield elements, illness resistance, and stress tolerance — this expertise has the potential to boost productiveness and resilience, significantly underneath difficult environmental situations pushed by local weather variability.
Furthermore, the system’s broad host vary and adaptableness might allow fast deployment in orphan crops and regionally necessary cereals the place typical transformation strategies stay elusive. This democratization of genome modifying expertise might empower breeders and researchers in creating international locations, fostering agricultural innovation tailor-made to native challenges and useful resource constraints.
Regardless of these promising advances, additional analysis will likely be important to optimize the supply effectivity throughout completely different genotypes and environmental situations. Understanding the interaction between viral an infection dynamics, plant immune responses, and modifying outcomes will assist in refining this platform and broadening its applicability. Moreover, complete off-target analyses and long-term area evaluations will assist validate the protection and stability of edited vegetation produced by way of this method.
The demonstration of heritable, non-transgenic genome modifying by way of a negative-strand RNA virus vector marks a major milestone in plant biotechnology. By marrying superior viral vector engineering with the precision of CRISPR–Cas methods and the pure mobility of tRNA sequences, the researchers have created a flexible, extremely environment friendly platform that would basically rework crop enchancment methods worldwide. This innovation exemplifies how leveraging refined organic data and molecular instruments converges to beat longstanding challenges, paving the way in which for a brand new period of sustainable, exact, and accessible plant genome modifying.
As the worldwide neighborhood faces escalating calls for for meals and environmental pressures, such pioneering work underscores the very important position of cutting-edge biotechnology in securing future agricultural resilience. The deployment of BYSMV-based genome modifying in wheat is greater than a technological breakthrough; it represents a paradigm shift towards fast, non-transgenic, heritable trait modification with monumental potential to reshape how crops are bred within the a long time to return.
Topic of Analysis: Genome modifying in wheat utilizing a negative-strand RNA virus vector system.
Article Title: Transgene- and tissue culture-free heritable genome modifying utilizing RNA virus-based supply in wheat.
Article References:
Qiao, JH., Zang, Y., Gao, Q. et al. Transgene- and tissue culture-free heritable genome modifying utilizing RNA virus-based supply in wheat. Nat. Vegetation (2025). https://doi.org/10.1038/s41477-025-02023-8
Picture Credit: AI Generated
Tags: barley yellow striate mosaic viruschallenges in plant genome editingCRISPR Cas9 supply systemefficient gene modifying in cropsheritable genetic modificationsmonocot transformation techniquesnon-transgenic genome engineeringnovel strategies in agricultural geneticsplant biotechnology advancementsRNA virus vector in plantsstable genome modificationswheat genome modifying