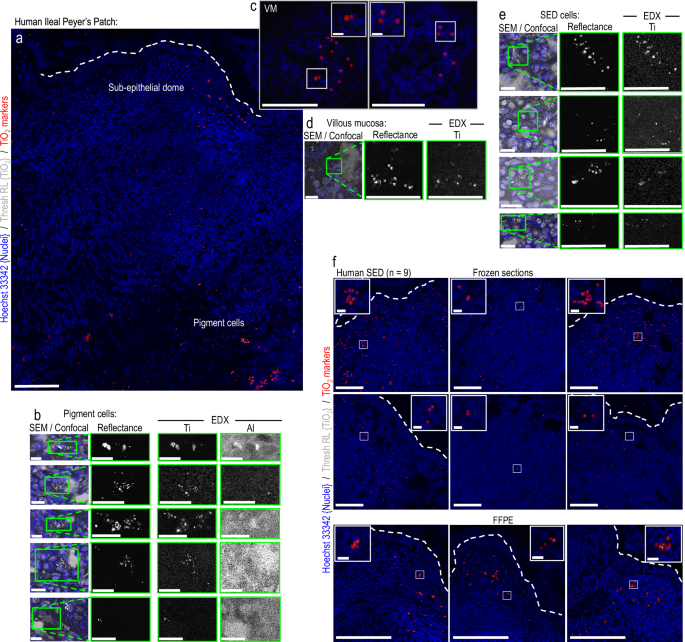
Think about the magnificent glaciers of Greenland, the everlasting snow of the Tibetan excessive mountains, and the completely ice-cold groundwater in Finland. As chilly and delightful as these are, for the structural biologist Kirill Kovalev, they’re extra importantly residence to uncommon molecules that might management mind cells’ exercise.
Kovalev, EIPOD Postdoctoral Fellow at EMBL Hamburg’s Schneider Group and EMBL-EBI’s Bateman Group, is a physicist keen about fixing organic issues. He’s significantly hooked by rhodopsins, a bunch of colourful proteins that allow aquatic microorganisms to harness daylight for power.
“In my work, I seek for uncommon rhodopsins and attempt to perceive what they do,” mentioned Kovalev. “Such molecules may have undiscovered features that we may gain advantage from.”
Some rhodopsins have already been modified to function light-operated switches for electrical exercise in cells. This system, known as optogenetics, is utilized by neuroscientists to selectively management neuronal exercise throughout experiments. Rhodopsins with different talents, equivalent to enzymatic exercise, could possibly be used to regulate chemical reactions with mild, for instance.
Having studied rhodopsins for years, Kovalev thought he knew them inside out—till he found a brand new, obscure group of rhodopsins that have been not like something he had seen earlier than.
Because it usually occurs in science, it began serendipitously. Whereas shopping on-line protein databases, Kovalev noticed an uncommon function frequent to microbial rhodopsins discovered solely in very chilly environments, equivalent to glaciers and excessive mountains.
“That is bizarre,” he thought. In any case, rhodopsins are one thing you usually discover in seas and lakes.
These cold-climate rhodopsins have been nearly similar to one another, although they developed hundreds of kilometers aside. This could not be a coincidence. They have to be important for surviving within the chilly, concluded Kovalev, and to acknowledge this, he named them “cryorhodopsins.”
Rhodopsins out of the blue
Kovalev wished to know extra: what these rhodopsins appear to be, how they work, and, particularly, what colour they’re.
Coloration is the important thing function of every rhodopsin. Most are pink-orange—they mirror pink and orange mild, and take up inexperienced and blue mild, which prompts them. Scientists try to create a palette of various coloured rhodopsins, so they might management neuronal exercise with extra precision. Blue rhodopsins have been particularly sought-after as a result of they’re activated by purple mild, which penetrates tissues extra deeply and non-invasively.
To Kovalev’s amazement, the cryorhodopsins he examined within the lab revealed an sudden variety of colours, and, most significantly, some have been blue. The work is revealed within the journal Science Advances.
The colour of every rhodopsin is set by its molecular construction, which dictates the wavelengths of sunshine it absorbs and displays. Any modifications on this construction can alter the colour.
“I can really inform what is going on on with cryorhodopsin just by its colour,” mentioned Kovalev.
Making use of superior structural biology methods, he found out that the key to the blue colour is similar uncommon structural function that he initially noticed within the protein databases.
“Now that we perceive what makes them blue, we are able to design artificial blue rhodopsins tailor-made to totally different functions,” mentioned Kovalev.
Subsequent, Kovalev’s collaborators examined cryorhodopsins in cultured mind cells. When cells expressing cryorhodopsins have been uncovered to UV mild, it induced electrical currents inside them. Curiously, if the researchers illuminated the cells proper afterwards with inexperienced mild, the cells grew to become extra excitable, whereas in the event that they used UV/purple mild as an alternative, it diminished the cells’ excitability.
“New optogenetic instruments to effectively swap the cell’s electrical exercise each ‘on’ and ‘off’ could be extremely helpful in analysis, biotechnology and medication,” mentioned Tobias Moser, Group Chief on the College Medical Heart Göttingen, who participated within the examine.
“For instance, in my group, we develop new optical cochlear implants for sufferers that may optogenetically restore listening to in sufferers. Creating the utility of such a multi-purpose rhodopsin for future functions is a vital job for the following research.”
“Our cryorhodopsins aren’t prepared for use as instruments but, however they’re a wonderful prototype. They’ve all the important thing options that, primarily based on our findings, could possibly be engineered to turn into simpler for optogenetics,” mentioned Kovalev.
Evolution’s UV mild protector
When uncovered to daylight even on a wet winter day in Hamburg, cryorhodopsins can sense UV mild, as proven utilizing superior spectroscopy by Kovalev’s collaborators from Goethe College Frankfurt, led by Josef Wachtveitl.
Wachtveitl’s staff confirmed that cryorhodopsins are in reality the slowest amongst all rhodopsins of their response to mild. This made the scientists suspect that these cryorhodopsins may act like photosensors, letting the microbes “see” UV mild—a property exceptional amongst different cryorhodopsins.
“Can they actually do this?” Kovalev stored asking himself. A typical sensor protein groups up with a messenger molecule that passes info from the cell membrane to the cell’s inside.
Kovalev grew extra satisfied, when collectively along with his collaborators from Alicante, Spain, and his EIPOD co-supervisor, Alex Bateman from EMBL-EBI, they observed that the cryorhodopsin gene is all the time accompanied by a gene encoding a tiny protein of unknown operate—doubtless inherited collectively, and probably functionally linked.
Kovalev questioned if this is likely to be the lacking messenger. Utilizing the AI software AlphaFold, the staff have been capable of present that 5 copies of the small protein would type a hoop and work together with the cryorhodopsin.
Based on their predictions, the small protein sits poised towards the cryorhodopsin contained in the cell. They consider that when cryorhodopsin detects UV mild, the small protein may depart to hold this info into the cell.
“It was fascinating to uncover a brand new mechanism by way of which the light-sensitive sign from cryorhodopsins could possibly be handed on to different elements of the cell. It’s all the time a thrill to be taught what the features are for uncharacterized proteins. Actually, we discover these proteins additionally in organisms that don’t comprise cryorhodopsin, maybe hinting at a a lot wider vary of jobs for these proteins.”
Why cryorhodopsins developed their astonishing twin operate—and why solely in chilly environments—stays a thriller.
“We suspect that cryorhodopsins developed their distinctive options not due to the chilly, however relatively to let microbes sense UV mild, which might be dangerous to them,” mentioned Kovalev.
“In chilly environments, equivalent to the highest of a mountain, micro organism face intense UV radiation. Cryorhodopsins may assist them sense it, so they might defend themselves. This speculation aligns properly with our findings.”
“Discovering extraordinary molecules like these would not be attainable with out scientific expeditions to usually distant places, to check the diversifications of the organisms dwelling there,” added Kovalev. “We will be taught a lot from that!”
Distinctive strategy to distinctive molecules
To disclose the fascinating biology of cryorhodopsins, Kovalev and his collaborators needed to overcome a number of technical challenges.
One was that cryorhodopsins are practically similar in construction, and even a slight change within the place of a single atom may end up in totally different properties. Learning molecules at this degree of element requires going past commonplace experimental strategies.
Kovalev utilized a 4D structural biology strategy, combining X-ray crystallography at EMBL Hamburg beamline P14 and cryo-electron microscopy (cryo-EM) within the group of Albert Guskov in Groningen, Netherlands, with protein activation by mild.
“I really selected to do my postdoc at EMBL Hamburg, due to the distinctive beamline setup that made my undertaking attainable,” mentioned Kovalev.
“The entire P14 beamline staff labored collectively to tailor the setup to my experiments—I am very grateful for his or her assist.”
One other problem was that cryorhodopsins are extraordinarily delicate to mild. Because of this, Kovalev’s collaborators needed to be taught to work with the samples in nearly full darkness.
Extra info:
Gerrit Lamm et al, CryoRhodopsins: a complete characterization of a bunch of microbial rhodopsins from chilly environments, Science Advances (2025). DOI: 10.1126/sciadv.adv1015. www.science.org/doi/10.1126/sciadv.adv1015
Offered by
European Molecular Biology Laboratory
Quotation:
Uncommon blue proteins from cold-adapted microbes may function prototypes for molecular on-off switches (2025, July 4)
retrieved 6 July 2025
from https://phys.org/information/2025-07-rare-blue-proteins-cold-microbes.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.