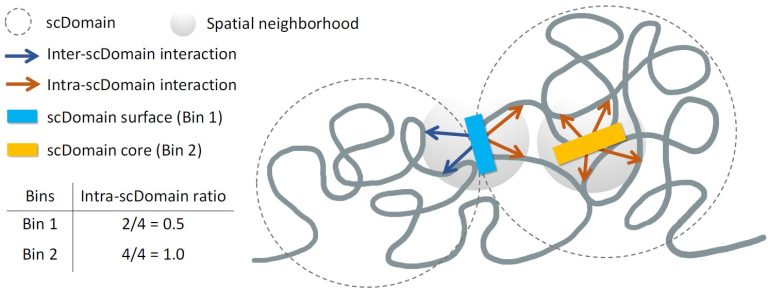
Ros Deegan is an business chief and the CEO of OMass Therapeutics, an Oxford-based biotechnology firm discovering medicines towards highly-validated goal ecosystems, resembling membrane proteins or intracellular complexes.
OMass’s MC2 program focusing on Congenital Adrenal Hyperplasia, or CAH, is ready to enter the clinic this 12 months and in our interview at present she walks me via how the corporate is getting ready for his or her trials, how their strategy differs to present remedy choices, and the impression on affected person lives they hope to have. A frontrunner within the UK biopharma business, she additionally explains the strengths of the UK business and the way it can keep an edge within the face of competitors from Europe and the US.
01:07 Meet Ros Deegan
02:36 Cambridge and INSEAD
04:24 Constructing business expertise
05:16 Biotech within the US vs Biotech within the UK
06:19 OMass: mission and ambition
07:16 Funding the mission
08:18 The significance of partnerships
09:17 Business and authorities
10:37 OMass and CAH
13:11 The differentiator for affected person outcomes
14:49 Into the clinic in 2025
16:31 CAH sufferers and affected person teams
19:29 The significance of hiring the correct individuals
20:55 The Oxford ‘model’ in biotechnology
22:17 Making ready for the clinic
23:43 Regulation and manufacturing
26:17 Partnering with high pharma
27:35 The significance of constructing worth
28:41 How the UK can compete on a world scale
30:39 What’s subsequent for OMass
32:59 The way forward for focusing on uncommon illness
Enthusiastic about being a sponsor of an episode of our podcast? Uncover how one can become involved right here!
Keep up to date by subscribing to our publication
To dive deeper into the subject:
Constructing business expertise
Biotech within the US vs Biotech within the UK
OMass: mission and ambition
The significance of partnerships
The differentiator for affected person outc
CAH sufferers and affected person teams
The significance of hiring the correct individuals
The Oxford ‘model’ in biotechnology
Regulation and manufacturing
Partnering with high pharma
The significance of constructing worth
How the UK can compete on a world scale
The way forward for focusing on uncommon illness