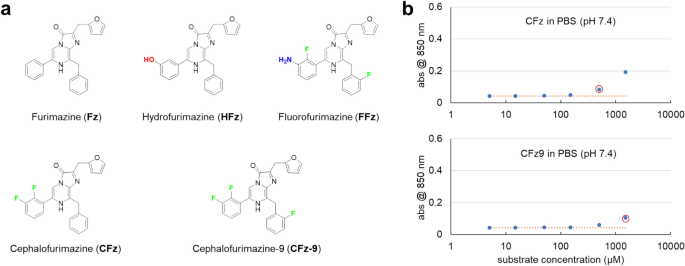
CFz9 with improved solubility for in vivo BLI purposes
CFz (Fig. 1a) was beforehand developed as an improved NanoLuc substrate for non-invasive imaging in animal brains16. For repeated, longitudinal BLI purposes, a normal dose of 1.3 μmol CFz with 13 mg P-407 solubilizer/excipient was beneficial, as day by day i.p. injections for as much as 5 days exhibited no observable toxicity in mice. In distinction, the maximal dose of 4.2 μmol CFz (with 20 mg P-407 in 500 μL Dulbecco’s Phosphate-Buffered Saline (DPBS)), a excessive dose that produces 6.5-fold brighter bioluminescence from the mind than the 1.3 μmol commonplace dose, was restricted to occasional single use, as histopathology evaluation revealed that 3 day by day injections at this dose led to organ injury. This toxicity was seemingly a mixed impact of each substrate and excipient16. It was noticed that 4.2 μmol of CFz with 20 mg P-407 approached the higher solubility restrict in 500 μL DPBS, evidenced by a small quantity of CFz residue after reconstitution of the injectable resolution beneath these situations16. Due to this fact, a NanoLuc substrate with related mind exercise as CFz and improved solubility might scale back excipient necessities and enhance tolerability, and thus can be extremely fascinating for longitudinal NanoLuc-based imaging in vivo.
Throughout CFz growth16, we synthesized a structural analogue with a further fluorine on the C8-substituted benzyl ring (Fig. 1a). This analogue, which we now designate as cephalofurimazine-9 (CFz9), demonstrated related in-brain BLI brightness on the identical dose as CFz, indicating comparable molar brightness within the animal mind. CFz fairly than CFz9 was chosen for additional characterization on the time beneath a conservative method of decreasing structural variations from the unique Fz substrate and pure CTZ analog. Nonetheless, in mild of the contribution of excipient to dose-limiting toxicity in CFz formulations16, we re-evaluated CFz9 for solubility. Utilizing absorbance at 850 nm as an point out of resolution turbidity, we discovered that CFz9 displays greater than threefold improved solubility in comparison with CFz in PBS with none excipient (Fig. 1b). We additionally discovered {that a} excessive dose (4.2 µmol) of CFz9 could possibly be solubilized by 12 mg melted P-407, in comparison with the 20 mg P-407 required for CFz. Consequently, we chosen CFz9 for additional research and carried out a reconstitution buffer display screen to optimize an injectable formulation for in vivo BLI.
Screening reconstitution buffers for CFz9 formulation with fascinating stability
To arrange injectable formulations, Fz analogues had been mixed with P-407 to present a yellow cake that was steady for long-term storage and prepared for reconstitution with buffers12,16. Beforehand, DPBS was used for reconstitution as a consequence of its physiological pH vary, biocompatibility, and cost-effectiveness. Nonetheless, a notable coloration change was noticed after 2 h of CFz reconstitution, suggesting instability in DBPS and necessitating speedy use of reconstituted CFz throughout animal BLI experiments. Bettering stability post-reconstitution can be extremely fascinating, by providing customers larger flexibility in dealing with and enhancing reproducibility throughout completely different operators.
As buffer traits comparable to pH, ionic energy, and element focus might have an effect on the chemical stability of oxidation-sensitive Fz analogues, we systematically evaluated widespread injectable reconstitution buffers. We examined a set of commonly-used laboratory buffers based mostly on their comfort, accessibility, and established biocompatibility in preclinical research (Fig. 2). Strong truffles of CFz9 and P-407 had been reconstituted utilizing the completely different buffers, adopted by LC–MS characterization of the reconstituted formulations. Two key parameters had been used to evaluate luciferin stability: “normalized peak space” represented the amount of luciferin remaining in resolution, with its lower indicating potential precipitation, whereas “purity” indicated the share of the intact luciferin peak relative to whole peaks recognized, revealing the proportion of undegraded luciferin. CFz9/P-407 reconstituted in DPBS (pH 7.0) exhibited poor solubility and stability over 2 h, with lower than 50% of CFz9 remaining soluble and with purity dropping beneath 80% within the soluble fraction (Fig. 2a, c), much like earlier observations with CFz. In distinction, water or saline reconstituted the CFz9/P-407 combination effectively, exhibiting negligible precipitation for six h and sustaining practically 100% purity (Fig. 2a, c). The comparatively poor efficiency of DPBS could point out a possible oxidative impact of phosphate on reconstituted CFz9 stability. Solutol HS15, a non-ionic surfactant widespread in pharmaceutical formulations19, additionally preserved luciferin stability, whereas glycerol20 proved insufficient for solubilizing hydrophobic CFz9, with over 50% precipitation noticed (Fig. 2a, c).
Reconstitution buffer screening for CFz9 formulation. (a,b) Evaluation of CFz9 solubility and stability in widespread aqueous options (a) and buffered techniques (b). Left, solubility measured as whole dissolved CFz9 quantified by LC–MS peak space; Proper, stability expressed as the share of intact CFz9 relative to all recognized peaks (purity). c, Comparative evaluation of CFz9 solubility and stability throughout examined situations from a and b at 6 h post-reconstitution. The purple field signifies the optimum buffer situations chosen for in vivo characterization.
Despite the fact that water or saline proved environment friendly in sustaining the soundness of reconstituted CFz9, for in vivo purposes, buffered techniques with pH near physiological situations are usually most well-liked, as they assist keep homeostasis post-injection. We subsequently evaluated varied generally used buffered techniques. Amongst acetate buffer (pH 5.2), bicarbonate buffer (pH 9.0), citrate buffer (pH 6.0), and HEPES buffer (pH 7.8), solely bicarbonate exhibited a big destabilizing impact on the CFz9/P-407 formulation, evidenced by a dramatic lower in soluble CFz9 after 3 h, with the purity of the remaining soluble fraction declining beneath 80% (Fig. 2b, c). We additionally investigated Tris buffer throughout varied pH situations and noticed a optimistic correlation between growing pH and enhanced stability of the CFz9/P-407 formulation (Fig. 2b, c). Amongst all examined buffered options, 100 mM Tris buffer (pH 8.0) demonstrated optimum stability traits, the place negligible CFz9 loss occurred over 6 h, with the purity of soluble compositions remaining above 90% (Fig. 2b, c). Prolonged stability research of this buffer situation revealed that after 10 h, roughly 70% of CFz9 remained in resolution, with the purity of soluble contents sustaining round 80% (Fig. S1a). Notable coloration adjustments had been noticed within the reconstituted resolution after 6 h, progressively darkening from mild yellow to black over 24 h post-reconstitution (Fig. S1b). Primarily based on these observations, we advocate utilizing the CFz9 formulation inside 6 h of reconstitution with 100 mM Tris buffer (pH 8.0), whereas the answer maintains its preliminary yellow or mild orange coloration.
We carried out an analogous reconstitution buffer display screen for FFz12, an amino-modified spinoff of Fz with considerably improved water solubility (Fig. 1a). Total, the variations between varied buffers had been much less pronounced for FFz, seemingly as a consequence of its enhanced water solubility and subsequently larger tendency to stay soluble within the formulated resolution. However, some developments noticed within the CFz9 buffer display screen continued, such because the stabilizing results of water and saline, and the destabilizing results of DPBS, bicarbonate, and HEPES buffers. Relating to the impression of various pH values in Tris buffer, FFz exhibited related peak depth and purity after 6 h throughout the pH vary (Fig. S2).
Toxicity research of the optimized injectable CFz9 formulation
Subsequent, to evaluate the security of the optimized CFz9 formulation, we carried out a complete blood chemistry and histopathological research. Male C57BL/6 mice (6–8 weeks outdated) had been injected with both automobile alone (P-407 in Tris buffer) or formulated CFz9 resolution at low dose (1.3 μmol CFz9 with 3.7 mg P-407 in 155 μL Tris buffer) or excessive dose (4.2 μmol CFz9 with 12 mg P-407 in 500 μL Tris buffer). Mice receiving low doses acquired three or 5 day by day injections, whereas these receiving excessive doses acquired both a single injection or three day by day injections. Physique weights had been monitored all through the research (Fig. 3a), and mice had been euthanized following their closing injection. Blood was collected for chemistry evaluation, and organs had been harvested for histopathological examination (Fig. 3b).
In vivo toxicity analysis of formulated CFz9. (a), Physique weight monitoring following i.p. administration of formulated CFz9 or P-407 automobile management. (b), Histology evaluation of organs with consultant micrographs from three mice per group. No apparent tissue injury was noticed within the examined tissues. Scale bars, 100 μm.
Whereas a lot of the blood chemistry parameters stay inside regular ranges, two indicators—whole ldl cholesterol and triglycerides—confirmed notable alterations, suggesting dyslipidemia (Desk S1). These parameters had been usually elevated throughout all remedy teams, with mice receiving three repeated injections of both high-dose automobile (12 mg P-407 alone) or formulated CFz9 (4.2 µmol CFz9 with 12 mg P-407) exhibiting markedly elevated ranges. The same pattern between these two teams prompt that P-407, fairly than CFz9, was the first reason behind dyslipidemia. This remark aligns with our earlier findings16and is per reported correlations between i.p. administered P-407 and elevated plasma ldl cholesterol and triglyceride concentrations in lab animals21,22.
One other parameter of curiosity was whole bilirubin (T-bil), which might be transiently elevated by drugs or can point out extra everlasting liver injury. Evaluation throughout vehicle-only, low-dose CFz9 (1.3 μmol), and high-dose CFz9 (4.2 µmol) teams revealed a dose-dependent rise in T-bil. Nonetheless, these results seemed to be transient fairly than as a consequence of long-term injury, as T-bil elevation didn’t correlate with the cumulative variety of doses administered (Desk S1). This interpretation was additional supported by the remark that the mouse physique weights remained inside acceptable ranges all through the toxicity research (Fig. 3a), and the absence of abnormalities in histopathological research, significantly in liver tissue sections (Fig. 3b). Total, regardless of these adjustments in blood chemistry, no vital or everlasting organ injury was noticed, an enchancment from the CFz formulation. These findings point out that the improved aqueous solubility of CFz9, enhanced chemical stability supplied by the Tris buffer system, and diminished excipient amount resulted in a safer formulation for repeated in vivo BLI purposes.
In vivo BLI with the optimized CFz9 formulation
With the optimized CFz9 formulation, we proceeded to judge its in vivo BLI efficiency by evaluating it to established optimum NanoLuc substrates. FFz, recognized for its optimum solubility regardless of restricted mind permeability, has been established as top-of-the-line substrates for BLI purposes exterior the mind12,23,24. Formulated FFz has lately develop into commercially accessible and has been extensively adopted for in vivo NanoLuc-based BLI research. When the identical group of mice expressing Antares, the red-shifted NanoLuc-based reporter, within the liver (pushed by Alb-cre, by which the Cre recombinase is expressed beneath the management of the albumin promoter, which is particularly lively in hepatocytes) was injected with both formulated FFz or CFz9 on completely different days for direct comparability, CFz9 displayed an analogous peak sign to FFz, though the sign decayed barely sooner (Fig. 4a, b). Thus, with the optimized formulation, CFz9 displays comparable in vivo BLI efficiency to the commercially accessible FFz.
In vivo characterization of formulated CFz9. (a-b), Comparability between formulated CFz9 and FFz in mouse liver. (a), Consultant pictures of in vivo BLI. (b), Left, bioluminescence depth within the mouse liver over time. Proper, Statistical evaluation of peak sign intensities. P values had been calculated utilizing paired t take a look at. (c-d), Characterization of post-reconstitution stability of formulated CFz9 for mind BLI. (c), Above, experimental design scheme. Beneath, consultant pictures of mind BLI. (d), Quantitation and statistical evaluation of peak sign intensities. P values had been calculated utilizing one-way ANOVA adopted by Dunnett’s publish hoc take a look at. Information are introduced as imply values ± s.e.m.
Having beforehand demonstrated that CFz9 and CFz exhibit comparable mind brightness at equal doses16, we subsequent evaluated the efficacy of optimized formulated CFz9 for mouse in-brain BLI, significantly specializing in the impact of its post-reconstitution stability on the in-brain BLI efficiency. The formulated CFz9 was reconstituted with 100 mM Tris (pH 8.0) buffer and allowed to face for a predetermined time intervals earlier than i.p. injection into transgenic mice expressing Antares within the mind (pushed by Camk2a-cre, which expresses the Cre recombinase beneath the management of calcium/calmodulin-dependent protein kinase II alpha promoter, which is predominantly lively in forebrain excitatory neurons). The identical cohort of mice acquired CFz9 formulations with various post-reconstitution stand-by occasions on completely different days (Fig. 4c). We then in contrast the ensuing BLI pictures to these obtained from the identical group of mice instantly injected with freshly ready CFz9 formulation. Our findings revealed that for as much as 6 h post-reconstitution, CFz9 maintained its total BLI brightness with out vital lower (Fig. 4c, d), aligning with our in vitro characterization which indicated stability of the reconstituted CFz9 formulation for as much as 6 h (Fig. S1b). Nonetheless, the BLI sign started to say no 8 h after CFz9 reconstitution and was diminished to 25% when the post-reconstitution time was prolonged to 24 h, suggesting that the formulation needs to be used inside 6 h of reconstitution for optimum efficiency.