By Aimee Raleigh, Principal at Atlas Enterprise, as a part of the From The Trenches function of LifeSciVC
Drug discovery and growth is a protracted, and infrequently fraught, journey – it sometimes takes greater than a decade to progress from concept to authorised drug, and fewer than 10% of medication that enter the clinic reach reaching commercialization. Creating new medicine for sufferers is definitely not for the faint of coronary heart. Behind each story of a drug authorised there are numerous tales of challenges, whether or not it was early know-how that didn’t scale, early compounds that proved poisonous in IND-enabling research, lack of scientific efficacy that wasn’t predicted by preclinical fashions, and lots of extra. The highway to success is rarely easy and infrequently takes a few years to ascertain traction. As an business we have to be steadfast even within the face of utmost doubt or hardship. That is a part of what makes us nice.
Nevertheless, there are occasions when refusing to cease and re-evaluate is to our drawback, particularly if our (and our groups’) bandwidth may be higher spent pursuing different potential therapies. I notice this second in time is very jarring to be discussing failure, when so lots of our core establishments for novel thesis era (NIH), drug growth and approval (FDA), and the fundamental tenets of our biotech economic system are in flux. However I consider the teachings on “failing quick” apply in any circumstance, and now greater than ever are essential as we take into consideration guaranteeing enduring success for our ecosystem.
How can we outline failure and success? How can we prospectively arrange frameworks for decision-making to maintain us trustworthy when a number of experiments learn out with grey or unfavourable knowledge? When can we collectively determine to close down a program? Each workforce goes by way of durations of doubt, and the choice of when to cease and when to persevere is exclusive to every circumstance. That stated, there are learnings we are able to draw from cumulative knowledge. For as we speak’s put up I’m honored to have collected suggestions from colleagues who’ve encountered the query of “ought to we cease” earlier than, and have dealt with it with integrity and thoughtfulness:
- Alex Harding has beforehand written in regards to the choice to cease, whether or not in shutting down seed-stage newco Apneo Therapeutics (right here) or extra usually because it applies to the general public markets (right here)
- Sam Truex spoke in regards to the choice to close down Quench Therapeutics after failing to establish tractable chemical matter towards gasdermin D (right here)
- Abbas Kazimi has written about “failing quick” relating to pipeline program administration at Nimbus Therapeutics (right here, right here)
Whether or not throughout an exploratory newco construct, after a big Collection A financing, or for particular person applications inside an organization’s pipeline, there are a set of frameworks that may assist groups parse by way of tough choices. Whereas we gained’t be diving into any company-specific examples on this piece, I’ll share cumulative learnings and suggestions from Alex, Sam, and Abbas (and myself!) for how one can strategy decision-making in conditions the place the trail ahead is muddied.
Arrange expectations earlier than kicking off a brand new firm or program
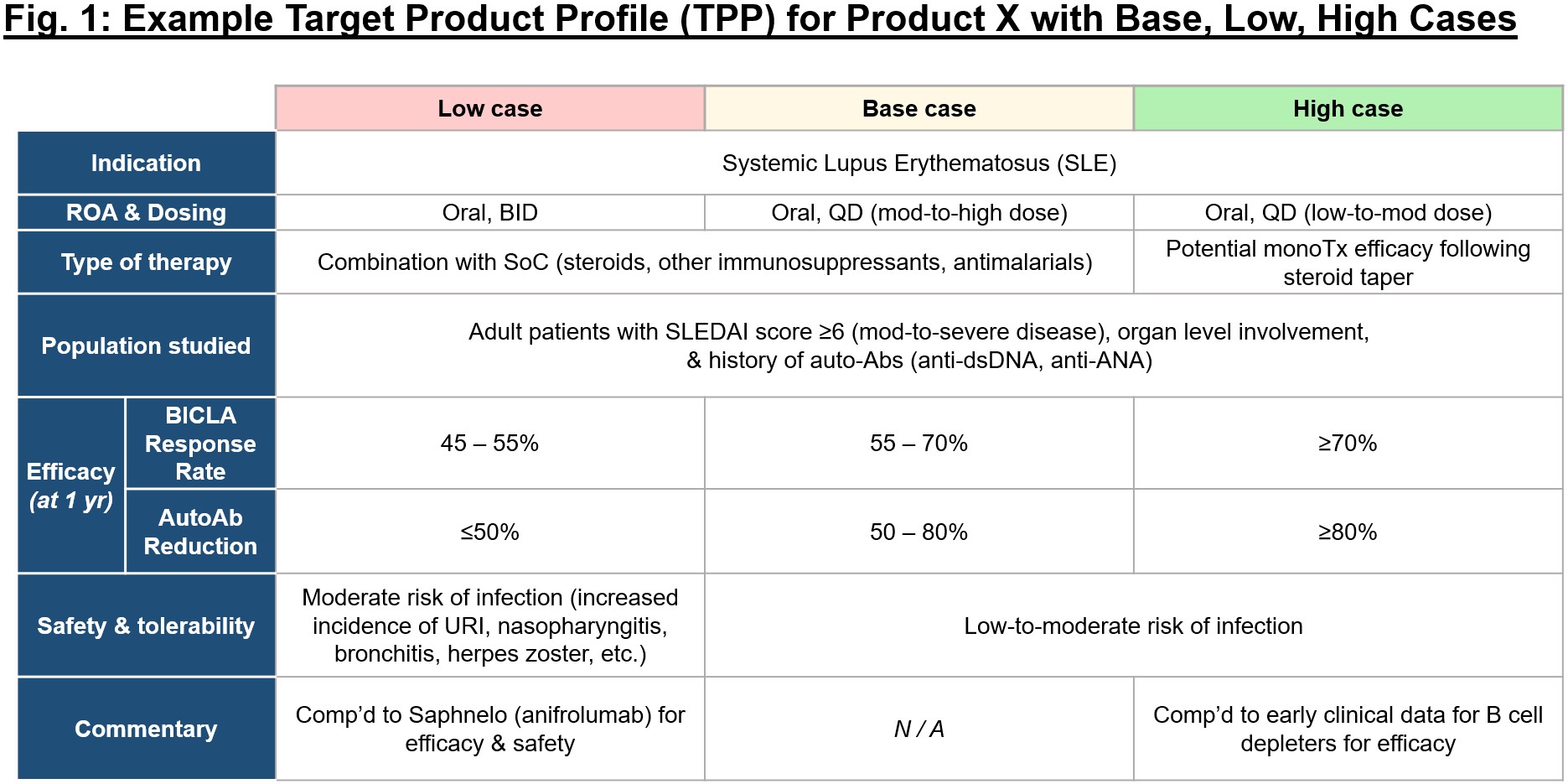
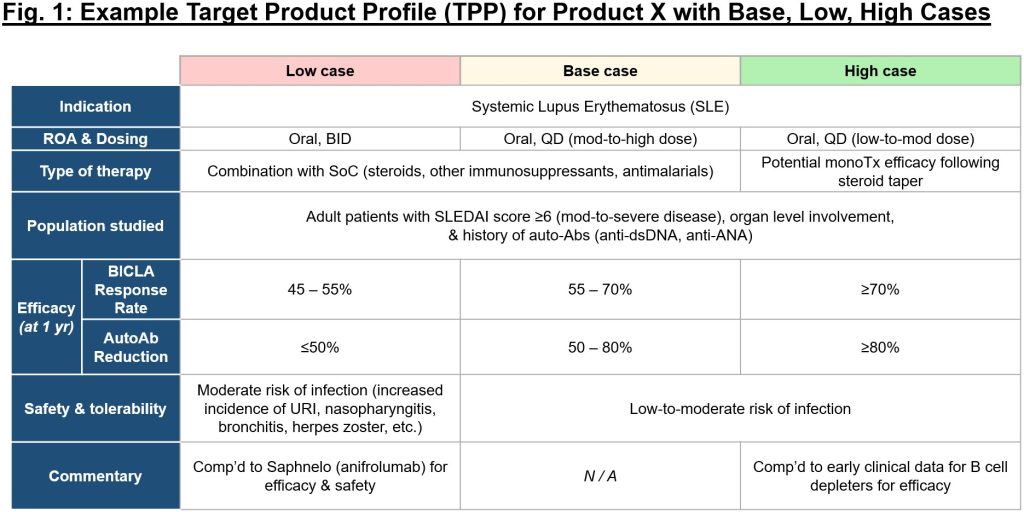
Failure is inherently outlined because the inverse of success, however so usually we don’t take the time to place pen to paper on what success appears to be like like for a person program or firm. It’s important to ascertain a rigorous analysis mindset early in new firm builds, and to constantly apply that mindset as objectively as potential. It takes time and thought, however earlier than any amount of cash is invested in a brand new concept, take into account mapping out this system(s) intimately, together with the primary 1-3 indications with highest mechanistic rationale. In constructing the goal product profile (TPP) for these, take into account what makes the mechanism uniquely suited to handle unmet wants in a given illness, and objectively map out what could be base, low, and excessive instances for this system within the context of the aggressive panorama (see Fig. 1 for an exemplary framework).
Because it’s usually exhausting to dive headfirst into complexity, Sam recommends beginning with the intense instances – what does a mediocre or poor profile seem like on this indication, and the way does that evaluate to a stellar profile? From there, you’ll be able to work out the achievable however nonetheless differentiated center floor, the “base case.” As soon as the theoretical profiles are established, you’ll be able to then cross-check inner and exterior knowledge over time to make sure the bottom case stays aggressive and helpful. Ensure to all the time keep conscientious of adjusting relative benchmarks, as you wouldn’t wish to put your head down in discovery for five years solely to appreciate the panorama on your prioritized indication has utterly modified and the bar is way larger than you initially thought. It’s vital to keep up self-discipline over conviction when evaluating your applications towards rivals – possibly you’ll be able to justify shifting ahead with a program that hits the low vs. base case on one parameter, however for all others this system ought to meet or exceed the bottom case. To stay goal, it’s useful to constantly pressure-test your view of the attractiveness of your program not solely with insiders (workforce, board, buyers), but additionally with exterior views (e.g., key opinion leaders).
This logic is a bit more easy for asset-centric firms, however what in case you are creating an early discovery-stage platform? Equally, it’s inspired to map out your key 1-3 applications earlier than even operating any experiments to validate your platform. Within the base case situation, how can your new know-how / modality uniquely allow the remedy of a illness past accessible (industrial and pipeline) therapies as we speak? In case you are at a loss for what these differentiated applications must be, it’s in all probability an indication that the platform idea wants some extra considering earlier than spending substantial funds to construct it out.
Lastly, maybe an apparent level however one which goes unaddressed in lots of new firms: nobody must be extra accustomed to “bear case” on your program than you. It’s a power and never a weak point to completely perceive the theoretical dangers of the mechanism or modality within the context of your lead indication. In reality, it’s protected to imagine that you’ll acquire credibility in being conscious of the dangers and expounding upon (and refuting) them soundly.
Map out potential outcomes earlier than an experimental readout
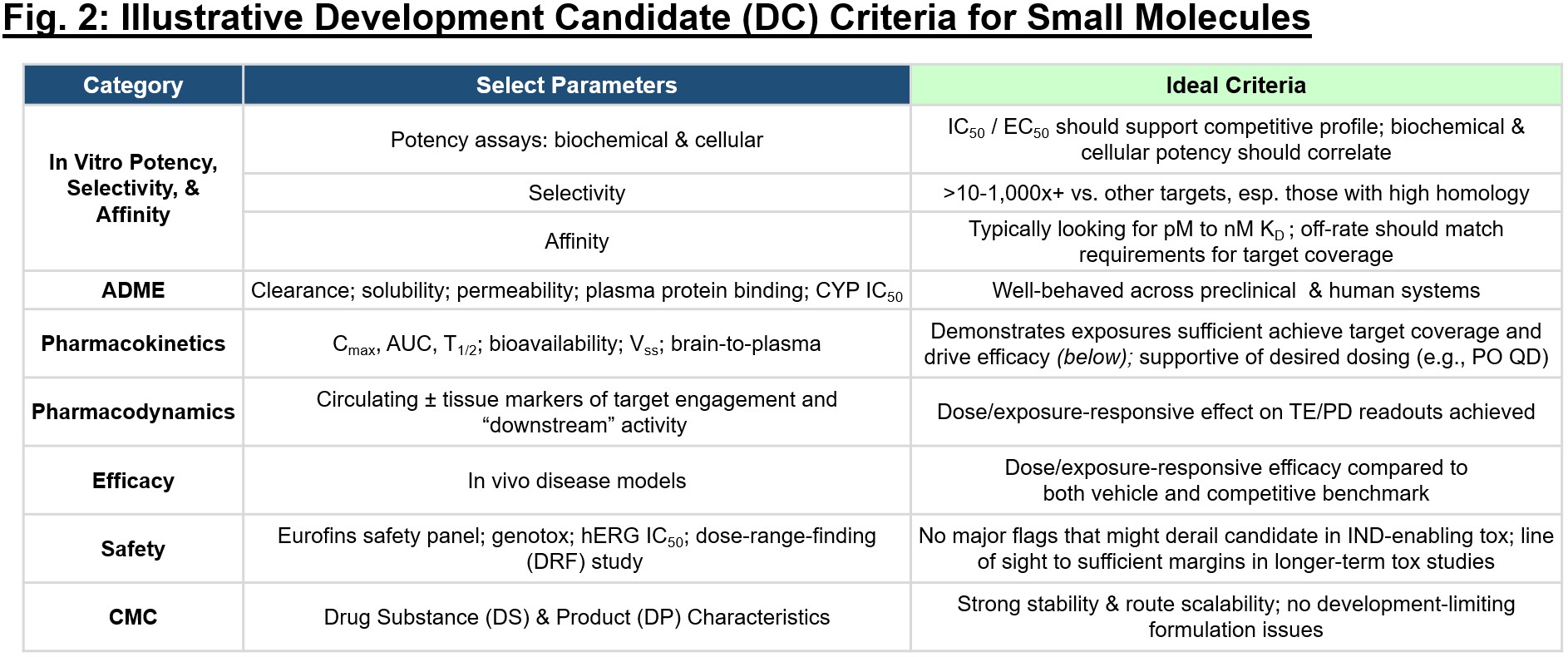
After all, the preliminary theoretical TPP will change over time as you generate knowledge and because the aggressive panorama shifts, but it surely’s vital to carry your self accountable to “what attractiveness like” as a program progresses. It’s additionally helpful when beginning a program in discovery to map the TPP again to a preclinical set of standards that’s required to uphold the “base case” within the scientific profile. Growth candidate (DC) standards (exemplary in Fig. 2) must be established to equally arrange threshold efficiency specs for a program which might be required to maneuver it ahead into IND-enabling research after which subsequently into the clinic. Each time you obtain a brand new piece of information, take into account it within the context of the DC standards and TPP – how does your estimation of this system stack up with this new knowledge in thoughts? Chances are you’ll change into extra bullish that the profile is aggressive and exceeding expectations, or you might determine {that a} sure limitation (e.g., slim tox margins, lack of compatibility with oral dosing) make the profile untenable for a given indication.
Sam recommends framing up objectives (annual, quarterly, and even per-experiment) based mostly on the assemble of “what would we like to have the ability to say about our program if it goes in addition to we may think about, and what is going to we’ve got confirmed?” It’s vital to put out expectations for achievement forward of a readout, to reduce bias that creeps in after we’ve got seen knowledge and know what’s or will not be possible. You’ll undoubtedly encounter hurdles – experiments which might be unfavourable or difficult to interpret. However establishing expectations prospectively permits you and your workforce to quickly pivot in a data-driven method and whereas protecting the big-picture DC standards and TPP in thoughts.
For a platform firm, take into account approaching each technological fork within the highway with the query “does this serve my applications and goal indications?” It could be tough to attract the road between what’s scientifically fascinating for the platform (continuous technological development and intelligent add-ons) vs. what is crucial to drive execution in direction of the clinic on your program. Very similar to you map out a DC guidelines and TPP, take into account making use of comparable logic for every experiment testing your platform:
- What is that this experiment testing?
- What does full success seem like, and the way does it allow my applications?
- If profitable, what are the following 1-3 experiments?
- How does this readout allow progress in direction of the clinic for my applications?
It may be simple to revert to letting the information drive choices, however in doing so we might stray away from the unique objectives and the therapeutic program in thoughts. For platform firms there are practically infinite levels of freedom, so there’s a steadiness between what’s “ok” for model 1.0 that allows progress in direction of clinic and what’s finest saved for model 2.0+ as soon as early de-risking is achieved.
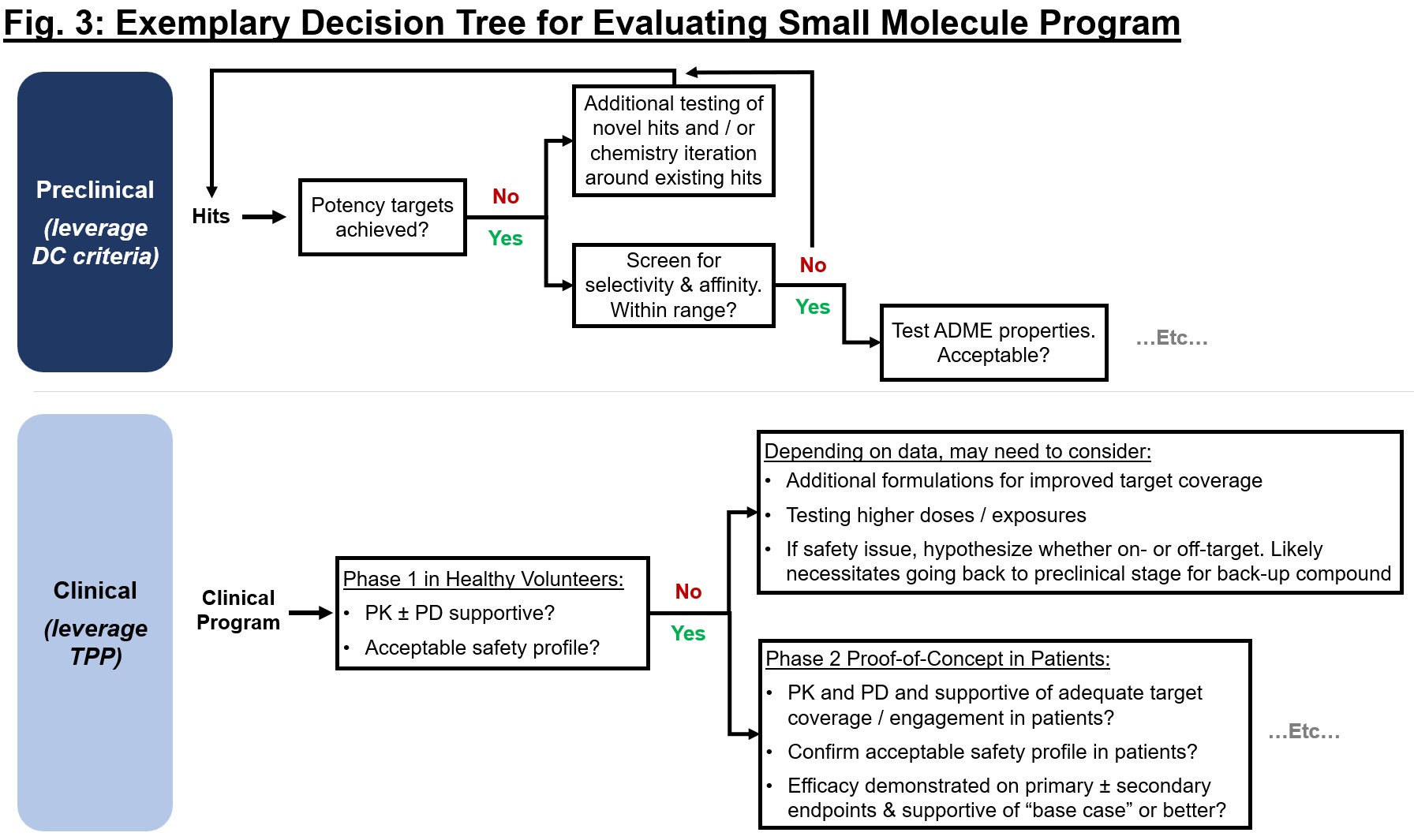
The DC standards and TPP can function inputs to distinct however associated choice frameworks that can be utilized to constantly consider preclinical or scientific readouts towards a prospectively outlined minimal viable profile (see Fig. 3). For every node within the choice tree, take time to map out expectations for essential readouts – what does the “go-forward” base case situation embody? An organization’s technique is finally influenced by these knowledge, so take into account implications for financing alternatives, strategic choices (extra pipeline, indication enlargement), and path to the clinic or eventual approval for the important thing readouts.
After all, nobody is suggesting a single experimental readout must be trigger for shutting down a program or firm. However what’s the proper standards? As Alex places it, it’s all about balancing curiosity and conviction with the likelihood of success, and which will look totally different for a given workforce, board, or firm. Historical past has taught us that in some cases (e.g., ALNY, TRIL, PCYC, LBPH), to surrender early would have meant forgoing unbelievable advances for sufferers. However these examples are sometimes exceptions to the rule, the place relentless persistence has paid off. For those who and your workforce have encountered experiment after experiment of grey knowledge, or have tried to fundraise for greater than a yr unsuccessfully, or are at a collective loss as to how one can progress a program, take a second of pause. Does the information (inner or exterior) let you know one thing totally different about your program or platform than your ingoing assumption? If that’s the case, is there a path ahead with a pivot, or is it time to reassess the trail ahead for this explicit thesis?
Finally, keep in mind that candor, objectivity, and proactivity are sometimes useful when murky knowledge emerges. It’s useful to floor everybody to the identical base case in order that when something sudden arises, stakeholders are finest located to judge it objectively and shortly decide the most effective path ahead. Drug discovery and growth is a workforce sport – groups and boards ought to collaboratively work collectively on all points of expectation-setting in addition to evaluation and choice as soon as the information emerges.
Admire that our cumulative definition of “failure” must be re-framed:
Now that we’ve arrange finest practices for rigorously evaluating a program or platform’s profile, it’s vital to acknowledge all of the nuanced the reason why remaining goal is so tough to do in actual life. Organising expectations after which lacking the mark may be existential, Alex mentions, particularly for an organization targeted on a single program or goal. Moreover, there could also be an uneven notion of dangers for an operator in comparison with different stakeholders, the place the previous might have devoted a few years to this sole endeavor and really feel a way of loss aversion when confronted with the potential of shutting it down.
All three of Alex, Sam, and Abbas have emphasised that the business ought to take into account how we outline and talk about success vs. failure. Rigorously creating a speculation and strategy to testing that speculation, whatever the finish end result, must be applauded as a hit. The one approach we are able to push our understanding of science and drugs ahead is to check these hypotheses and disseminate the readouts to the neighborhood. The place would the incretin area be if Novo hadn’t persevered in bettering the half-life of GLP-1 analogs when authentic molecules stalled? If Roche hadn’t first didn’t sluggish Alzheimer’s development with gantenerumab and crenezumab, would they’ve had the insights to develop trontinemab, a next-gen TfR1 shuttle conjugated to anti-amyloid? Numerous new applications are born out of hard-learned failures.
Moreover, it’s vital to dissociate any ego or private id from the end result of a well-planned experiment. Simply because a program or know-how fails to maneuver ahead doesn’t indicate the folks operating this system failed. In reality, in the event that they acquired to a “no-go” shortly, they made an enormous contribution to their workforce’s and the collective business’s data for a given goal or strategy. Abbas recommends cheering for your self and others if you determine to close a program down simply as a lot as when a program strikes ahead – finally our ecosystem is data-driven, and even unfavourable knowledge may help to advance data and future medicines. At Nimbus, they’ve a saying: “like this system, love the portfolio.” To let go of 1 program the place the thesis hasn’t panned out frees up bandwidth and sources to execute on applications that do. With this mentality in thoughts, over a consultant timeframe from 2016-2022, Nimbus shut down 70% of the applications it was engaged on for a wide range of causes: scientific, industrial, aggressive positioning, and so on. In acknowledging when a thesis wasn’t advancing, their workforce collectively re-deployed vitality and expertise to these applications that did, undoubtedly driving to the profitable TYK2 program (now within the palms of Takeda), WRN inhibitor, and extra.
It must be famous that success in “failing quick” is finest enabled when firm management, buyers, boards, and different stakeholders are all aligned on the acknowledged mission and expectation for “what attractiveness like.” This logic is finest framed forward of readouts and when firms have adequate runway, as nothing breeds poor choices like a restricted runway and lack of backup choices.
Each firm and state of affairs is exclusive, so there is no such thing as a proper or incorrect reply on when to maintain pushing vs. throw within the towel. Hopefully the frameworks listed below are helpful the following time you end up staring down that query. Finally we every have a hard and fast period of time to do some actual good for sufferers – how will you determine to spend it?
Thanks to Sam Truex, Alex Harding, and Abbas Kazimi for generously sharing their views and time for this text. Many thanks additionally to Akshay Vaishnaw for offering suggestions on this put up.