Brittberg, M., Recker, D., Ilgenfritz, J. & Saris, D. B. F. Matrix-applied characterised autologous cultured chondrocytes versus microfracture: five-year follow-up of a potential randomized trial. Am. J. Sports activities Med. 46, 1343–1351 (2018).
Schmidt, C. Gintuit cell remedy approval indicators shift at US regulator. Nat. Biotechnol. 30, 479–479 (2012).
Swan, M. Regular advance of stem cell therapies: report from the 2011 World Stem Cell Summit, Pasadena, California, October 3–5. Rejuvenation Res. 14, 699–704 (2011).
Carmeliet, P. & Jain, R. Okay. Angiogenesis in most cancers and different illnesses. Nature 407, 249–257 (2000).
Miller, J. S. et al. Fast casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Phelps, E. A. & Garcia, A. J. Replace on therapeutic vascularization methods. Regen. Med. 4, 65–80 (2009).
Gandhi, J. Okay., Opara, E. C. & Brey, E. M. Alginate-based methods for therapeutic vascularization. Ther. Deliv. 4, 327–341 (2013).
Bowers, D. T., Tune, W., Wang, L.-H. & Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 95, 131–151 (2019).
Gianni‐Barrera, R. et al. Therapeutic vascularization in regenerative drugs. Stem Cells Transl. Med. 9, 433–444 (2020).
Wilson, Z. E. et al. Inter-individual variability in ranges of human microsomal protein and hepatocellularity per gram of liver. Br. J. Clin. Pharmacol. 56, 433–440 (2003).
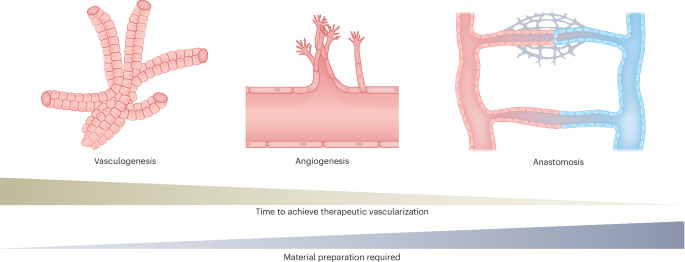
Risau, W. & Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 11, 73–91 (1995).
Risau, W. Mechanisms of angiogenesis. Nature 386, 671–674 (1997).
Zhang, B. et al. Microfabrication of AngioChip, a biodegradable polymer scaffold with microfluidic vasculature. Nat. Protoc. 13, 1793–1813 (2018).
Zhang, B. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 (2016).
Szklanny, A. A. et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Adv. Mater. 33, 2102661 (2021).
Wang, X. et al. Bettering islet engraftment by gene remedy. J. Transplant. 2011, e594851 (2011).
Ajioka, I. et al. Institution of heterotropic liver tissue mass with direct hyperlink to the host liver following implantation of hepatocytes transfected with vascular endothelial development issue gene in mice. Tissue Eng. 7, 335–344 (2001).
Vlahos, A. E. et al. Endothelialized collagen based mostly pseudo-islets allows tuneable subcutaneous diabetes remedy. Biomaterials 232, 119710 (2020).
Weaver, J. D. et al. Vasculogenic hydrogel enhances islet survival, engraftment, and performance in main extrahepatic websites. Sci. Adv. 3, e1700184 (2017).
Phelps, E. A., Templeman, Okay. L., Thulé, P. M. & García, A. J. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 5, 125–136 (2015).
Mirabella, T. et al. 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat. Biomed. Eng. 1, 0083 (2017).
Baranski, J. D. et al. Geometric management of vascular networks to reinforce engineered tissue integration and performance. Proc. Natl Acad. Sci. USA 110, 7586–7591 (2013).
Tune, W. et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 10, 4602 (2019).
Mastrullo, V., Cathery, W., Velliou, E., Madeddu, P. & Campagnolo, P. Angiogenesis in tissue engineering: as nature supposed? Entrance. Bioeng. Biotechnol. 2020, 188 (2020).
Goldie, L. C., Nix, M. Okay. & Hirschi, Okay. Okay. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 4, 257–263 (2008).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–966 (1997).
Asahara, T. et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18, 3964–3972 (1999).
Takahashi, T. et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 (1999).
De Spiegelaere, W. et al. Intussusceptive angiogenesis: a biologically related type of angiogenesis. J. Vasc. Res. 49, 390–404 (2012).
Rouwkema, J. & Khademhosseini, A. Vascularization and angiogenesis in tissue engineering: past creating static networks. Traits Biotechnol. 34, 733–745 (2016).
Burri, P. H., Hlushchuk, R. & Djonov, V. Intussusceptive angiogenesis: its emergence, its traits, and its significance. Dev. Dyn. 231, 474–488 (2004).
Hellström, M. et al. Dll4 signalling by means of Notch1 regulates formation of tip cells throughout angiogenesis. Nature 445, 776–780 (2007).
Roesel, J. F. & Nanney, L. B. Evaluation of differential cytokine results on angiogenesis utilizing an in vivo mannequin of cutaneous wound restore. J. Surg. Res. 58, 449–459 (1995).
Wei, L.-H. et al. Interleukin-6 promotes cervical tumor development by VEGF-dependent angiogenesis by way of a STAT3 pathway. Oncogene 22, 1517–1527 (2003).
Li, A., Dubey, S., Varney, M. L., Dave, B. J. & Singh, R. Okay. IL-8 instantly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases manufacturing and controlled angiogenesis. J. Immunol. 170, 3369–3376 (2003).
Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA 100, 2645–2650 (2003).
Miller, J. S. et al. Bioactive hydrogels constructed from step-growth derived PEG–peptide macromers. Biomaterials 31, 3736–3743 (2010).
Djonov, V., Baum, O. & Burri, P. H. Vascular reworking by intussusceptive angiogenesis. Cell Tissue Res. 314, 107–117 (2003).
Caduff, J. H., Fischer, L. C. & Burri, P. H. Scanning electron microscope examine of the creating microvasculature within the postnatal rat lung. Anat. Rec. 216, 154–164 (1986).
Grundmann, S., Piek, J. J., Pasterkamp, G. & Hoefer, I. E. Arteriogenesis: primary mechanisms and therapeutic stimulation. Eur. J. Clin. Make investments. 37, 755–766 (2007).
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6, 389–395 (2000).
Giacca, M. & Zacchigna, S. VEGF gene remedy: therapeutic angiogenesis within the clinic and past. Gene Ther. 19, 622–629 (2012).
Henry, T. D. et al. The VIVA trial. Circulation 107, 1359–1365 (2003).
Simón-Yarza, T. et al. Vascular endothelial development factor-delivery techniques for cardiac restore: an outline. Theranostics 2, 541–552 (2012).
Darden, J., Payne, L. B., Zhao, H. & Chappell, J. C. Extra vascular endothelial development factor-A disrupts pericyte recruitment throughout blood vessel formation. Angiogenesis 22, 167–183 (2018).
Blatchley, M. R., Corridor, F., Wang, S., Pruitt, H. C. & Gerecht, S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Sci. Adv. 5, eaau7518 (2019). This examine describes the mechanism by means of which hypoxia and the fabric properties of the matrix affect vasculogenesis.
Longchamp, A. et al. Amino acid restriction triggers angiogenesis by way of GCN2/ATF4 regulation of VEGF and H2S manufacturing. Cell 173, 117–129.e14 (2018).
Fiedler, J. et al. Practical microRNA library screening identifies the hypoxaMiR MiR-24 as a potent regulator of easy muscle cell proliferation and vascularization. Antioxid. Redox Sign. 21, 1167–1176 (2014).
Dou, C. et al. Graphene-based microRNA transfection blocks preosteoclast fusion to extend bone formation and vascularization. Adv. Sci. 5, 1700578 (2018).
Li, Y. et al. Injectable hydrogel with MSNs/microRNA-21-5p supply allows each immunomodification and enhanced angiogenesis for myocardial infarction remedy in pigs. Sci. Adv. 7, eabd6740 (2021).
Lamichhane, T. N., Leung, C. A., Douti, L. Y. & Jay, S. M. Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles by way of regulation of microRNAs and lengthy non-coding RNAs. Sci. Rep. 7, 13794 (2017).
Welten, S. M. J. et al. Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 will increase neovascularization and blood circulate restoration after ischemia. Circ. Res. 115, 696–708 (2014).
Liu, B. et al. Cardiac restoration by way of prolonged cell-free supply of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2, 293–303 (2018).
Kawamoto, A. et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107, 461–468 (2003).
Losordo, D. W. et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina. Circulation 115, 3165–3172 (2007).
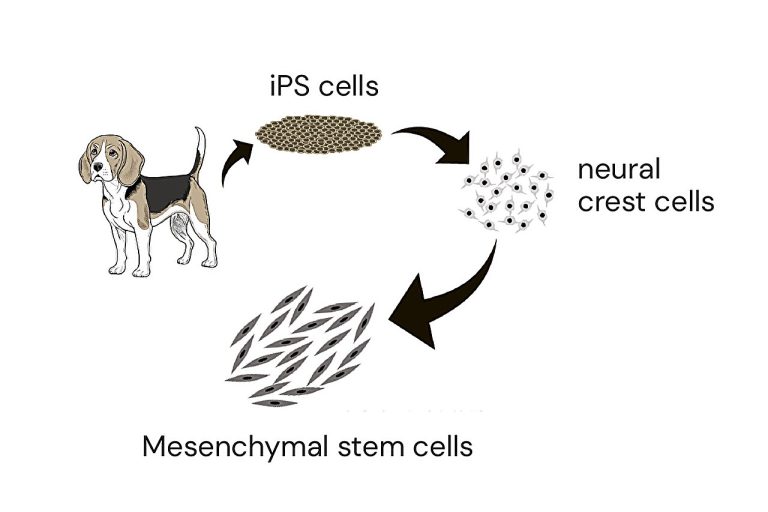
Calderon, G. A. et al. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels. Biomater. Sci. 5, 1652–1660 (2017).
Moon, J. J. et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 31, 3840–3847 (2010).
Pedowitz, N. J., Batt, A. R., Darabedian, N. & Pratt, M. R. MYPT1 O-GlcNAc modification regulates sphingosine-1-phosphate mediated contraction. Nat. Chem. Biol. 17, 169–177 (2021). This examine particulars how a specific post-translational modification impacts diabetic wound therapeutic.
Nadkarni, S. et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in being pregnant. Proc. Natl Acad. Sci. USA 113, E8415–E8424 (2016).
Mor, F., Quintana, F. J. & Cohen, I. R. Angiogenesis–irritation cross-talk: vascular endothelial development issue is secreted by activated T cells and induces Th1 polarization. J. Immunol. 172, 4618–4623 (2004).
Marek, N. et al. Elevated spontaneous manufacturing of VEGF by CD4+ T cells in sort 1 diabetes. Clin. Immunol. 137, 261–270 (2010).
Ribatti, D. & Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 13, 2822–2833 (2009).
Graney, P. L. et al. Macrophages of various phenotypes drive vascularization of engineered tissues. Sci. Adv. 6, eaay6391 (2020).
Fleischer, S., Tavakol, D. N. & Vunjak-Novakovic, G. From arteries to capillaries: approaches to engineering human vasculature. Adv. Funct. Mater. 30, 1910811 (2020).
Zhao, S. et al. Software of stem cells in engineered vascular graft and vascularized organs. Semin. Cell Dev. Biol. 144, 31–40 (2023).
Kumar, A. H. S. & Caplice, N. M. Medical potential of grownup vascular progenitor cells. Arter. Thromb. Vasc. Biol. 30, 1080–1087 (2010).
Bayraktutan, U. Endothelial progenitor cells: potential novel therapeutics for ischaemic stroke. Pharmacol. Res. 144, 181–191 (2019).
Nowbar, A. N. et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Br. Med. J. 348, g2688 (2014).
Krawiec, J. T. & Vorp, D. A. Grownup stem cell-based tissue engineered blood vessels: a evaluate. Biomaterials 33, 3388–3400 (2012).
Galat, V. et al. Transgene reactivation in induced pluripotent stem cell derivatives and reversion to pluripotency of induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Dev. 25, 1060–1072 (2016).
Hentze, H. et al. Teratoma formation by human embryonic stem cells: analysis of important parameters for future security research. Stem Cell Res. 2, 198–210 (2009).
Quint, C. et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl Acad. Sci. USA 108, 9214–9219 (2011).
Neff, L. P. et al. Vascular easy muscle enhances performance of tissue-engineered blood vessels in vivo. J. Vasc. Surg. 53, 426–434 (2011).
Kaushal, S. et al. Practical small-diameter neovessels created utilizing endothelial progenitor cells expanded ex vivo. Nat. Med. 7, 1035–1040 (2001).
He, H., Shirota, T., Yasui, H. & Matsuda, T. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential. J. Thorac. Cardiovasc. Surg. 126, 455–464 (2003).
Zhu, C. et al. Growth of anti-atherosclerotic tissue-engineered blood vessel by A20-regulated endothelial progenitor cells seeding decellularized vascular matrix. Biomaterials 29, 2628–2636 (2008).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived inhabitants. Nature 453, 524–528 (2008).
Bu, L. et al. Human ISL1 coronary heart progenitors generate various multipotent cardiovascular cell lineages. Nature 460, 113–117 (2009).
Ferreira, L. S. et al. Vascular progenitor cells remoted from human embryonic stem cells give rise to endothelial and easy muscle like cells and type vascular networks in vivo. Circ. Res. 101, 286–294 (2007).
Levenberg, S., Ferreira, L. S., Chen-Konak, L., Kraehenbuehl, T. P. & Langer, R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat. Protoc. 5, 1115–1126 (2010).
Hill, Okay. L. et al. Human embryonic stem cell-derived vascular progenitor cells able to endothelial and easy muscle cell operate. Exp. Hematol. 38, 246–257.e1 (2010).
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 4391–4396 (2002).
Cheung, C. & Sinha, S. Human embryonic stem cell-derived vascular easy muscle cells in therapeutic neovascularisation. J. Mol. Cell. Cardiol. 51, 651–664 (2011).
Sone, M. et al. Pathway for differentiation of human embryonic stem cells to vascular cell elements and their potential for vascular regeneration. Arter. Thromb. Vasc. Biol. 27, 2127–2134 (2007).
Taura, D. et al. Induction and isolation of vascular cells from human induced pluripotent stem cells—transient report. Arterioscler. Thromb. Vasc. Biol. 29, 1100–1103 (2009).
Patsch, C. et al. Era of vascular endothelial and easy muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003 (2015).
Sundaram, S., Echter, A., Sivarapatna, A., Qiu, C. & Niklason, L. Small-diameter vascular graft engineered utilizing human embryonic stem cell-derived mesenchymal cells. Tissue Eng. A 20, 740–750 (2014).
Wang, L. et al. Fabrication of tissue-engineered vascular grafts with stem cells and stem cell-derived vascular cells. Professional Opin. Biol. Ther. 16, 317–330 (2016).
Jang, S., Collin de l’Hortet, A. & Soto-Gutierrez, A. Induced pluripotent stem cell-derived endothelial cells: overview, present advances, functions, and future instructions. Am. J. Pathol. 189, 502–512 (2019).
Margariti, A. et al. Direct reprogramming of fibroblasts into endothelial cells able to angiogenesis and reendothelialization in tissue-engineered vessels. Proc. Natl Acad. Sci. USA 109, 13793–13798 (2012).
Shen, M., Quertermous, T., Fischbein, M. P. & Wu, J. C. Era of vascular easy muscle cells from induced pluripotent stem cells: strategies, functions, and issues. Circ. Res. 128, 670–686 (2021).
Generali, M. et al. Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells. Acta Biomater. 97, 333–343 (2019).
Abaci, H. E. et al. Human pores and skin constructs with spatially managed vasculature utilizing main and iPSC-derived endothelial cells. Adv. Healthc. Mater. 5, 1800–1807 (2016).
Ciampi, O. et al. Engineering the vasculature of decellularized rat kidney scaffolds utilizing human induced pluripotent stem cell-derived endothelial cells. Sci. Rep. 9, 8001 (2019).
Wang, Y. Y. et al. Engineering vascular tissue with practical easy muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials 35, 8960–8969 (2014).
Luo, J. et al. Tissue-engineered vascular grafts with superior mechanical power from human iPSCs. Cell Stem Cell 26, 251–261.e8 (2020).
Gui, L. et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102, 120–129 (2016).
Atchison, L. et al. iPSC-derived endothelial cells have an effect on vascular operate in a tissue-engineered blood vessel mannequin of Hutchinson–Gilford progeria syndrome. Stem Cell Rep. 14, 325–337 (2020).
Owens, G. Okay., Kumar, M. S. & Wamhoff, B. R. Molecular regulation of vascular easy muscle cell differentiation in improvement and illness. Physiol. Rev. 84, 767–801 (2004).
Majesky, M. W. Developmental foundation of vascular easy muscle variety. Arter. Thromb. Vasc. Biol. 27, 1248–1258 (2007).
Turner, M. et al. Towards the event of a world induced pluripotent stem cell library. Cell Stem Cell 13, 382–384 (2013).
Wilmut, I. et al. Growth of a world community of induced pluripotent stem cell haplobanks. Regen. Med. 10, 235–238 (2015).
Lee, S. et al. Repurposing the twine blood financial institution for haplobanking of HLA-homozygous iPSCs and their usefulness to a number of populations. Stem Cells 36, 1552–1566 (2018).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long run in absolutely immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42, 413–423 (2024).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 (2017).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in absolutely immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A. & Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological circumstances. Biomed. Res. Int. 2014, 756078 (2014).
Senger, D. R. & Davis, G. E. Angiogenesis. Chilly Spring Harb. Perspect. Biol. 3, a005090 (2011).
Stupack, D. G. & Cheresh, D. A. ECM reworking regulates angiogenesis: endothelial integrins search for new ligands. Sci. STKE 2002, pe7 (2002).
Ouyang, L. et al. MMP‑delicate PEG hydrogel modified with RGD promotes bFGF, VEGF and EPC‑mediated angiogenesis. Exp. Ther. Med. 18, 2933–2941 (2019).
Nemati, S. et al. Alginate–gelatin encapsulation of human endothelial cells promoted angiogenesis in in vivo and in vitro milieu. Biotechnol. Bioeng. 114, 2920–2930 (2017).
Li, Z. et al. Injectable gelatin by-product hydrogels with sustained vascular endothelial development issue launch for induced angiogenesis. Acta Biomater. 13, 88–100 (2015).
Search engine optimization, Y., Jung, Y. & Kim, S. H. Decellularized coronary heart ECM hydrogel utilizing supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 67, 270–281 (2018).
Griffin, M. E., Sorum, A. W., Miller, G. M., Goddard, W. A. & Hsieh-Wilson, L. C. Sulfated glycans have interaction the Ang–Tie pathway to control vascular improvement. Nat. Chem. Biol. 17, 178–186 (2021).
Jia, J. et al. Evolutionarily conserved sequence motif evaluation guides improvement of chemically outlined hydrogels for therapeutic vascularization. Sci. Adv. 6, eaaz5894 (2020).
Ruehle, M. A. et al. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 6, eabb6351 (2020).
Mandrycky, C., Hadland, B. & Zheng, Y. 3D curvature-instructed endothelial circulate response and tissue vascularization. Sci. Adv. 6, eabb3629 (2020). This examine quantifies the impact of vessel geometry and perfusion-related forces on endothelial cells.
Lee, J. et al. Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat. Biomed. Eng. 5, 89–102 (2021).
Wolf, Okay. J., Weiss, J. D., Uzel, S. G. M., Skylar-Scott, M. A. & Lewis, J. A. Biomanufacturing human tissues by way of organ constructing blocks. Cell Stem Cell 29, 667–677 (2022).
Munarin, F., Kant, R. J., Rupert, C. E., Khoo, A. & Coulombe, Okay. L. Okay. Engineered human myocardium with native launch of angiogenic proteins improves vascularization and cardiac operate in injured rat hearts. Biomaterials 251, 120033 (2020).
Nih, L. R., Gojgini, S., Carmichael, S. T. & Segura, T. Twin-function injectable angiogenic biomaterial for the restore of mind tissue following stroke. Nat. Mater. 17, 642–651 (2018).
Lee, A. S. et al. Extended survival of transplanted stem cells after ischaemic damage by way of the gradual launch of pro-survival peptides from a collagen matrix. Nat. Biomed. Eng. 2, 104–113 (2018).
Aday, S. et al. Artificial microparticles conjugated with VEGF165 enhance the survival of endothelial progenitor cells by way of microRNA-17 inhibition. Nat. Commun. 8, 747 (2017).
Coindre, V. F., Carleton, M. M. & Sefton, M. V. Methacrylic acid copolymer coating enhances constructive reworking of polypropylene mesh by growing the vascular response. Adv. Healthc. Mater. 8, 1900667 (2019).
Coindre, V. F., Kinney, S. M. & Sefton, M. V. Methacrylic acid copolymer coating of polypropylene mesh chamber improves subcutaneous islet engraftment. Biomaterials 259, 120324 (2020).
Yu, Y. et al. Sulfated polysaccharide directs therapeutic angiogenesis by way of endogenous VEGF secretion of macrophages. Sci. Adv. 7, eabd8217 (2021).
Li, X. et al. Nanofiber-hydrogel composite-mediated angiogenesis for smooth tissue reconstruction. Sci. Transl. Med. 11, eaau6210 (2019).
Mazio, C. et al. Pre-vascularized dermis mannequin for quick and practical anastomosis with host vasculature. Biomaterials 192, 159–170 (2019).
Roberts, S. et al. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 17, 1154–1163 (2018). This examine describes the creation of partially ordered peptides for vascularizing supplies with tunable properties on the premise of ordered/disordered domains.
Wang, L. et al. Growth of a centrally vascularized tissue engineering bone graft with the distinctive core-shell composite construction for giant femoral bone defect remedy. Biomaterials 175, 44–60 (2018).
Dor, Y. Conditional switching of VEGF offers new insights into grownup neovascularization and pro-angiogenic remedy. EMBO J. 21, 1939–1947 (2002).
Tafuro, S. et al. Inducible adeno-associated virus vectors promote practical angiogenesis in grownup organisms by way of regulated vascular endothelial development issue expression. Cardiovasc. Res. 83, 663–671 (2009).
Ozawa, C. R. et al. Microenvironmental VEGF focus, not complete dose, determines a threshold between regular and aberrant angiogenesis. J. Clin. Make investments. 113, 516–527 (2004).
Ozaki, H. et al. Intravitreal sustained launch of VEGF causes retinal neovascularization in rabbits and breakdown of the blood–retinal barrier in rabbits and primates. Exp. Eye Res. 64, 505–517 (1997).
Murphy, W. L., Peters, M. C., Kohn, D. H. & Mooney, D. J. Sustained launch of vascular endothelial development issue from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21, 2521–2527 (2000).
Lee, Okay. W. et al. Sustained launch of vascular endothelial development issue from calcium-induced alginate hydrogels strengthened by heparin and chitosan. Transplant. Proc. 36, 2464–2465 (2004).
Zisch, A. H. et al. Cell-demanded launch of VEGF from artificial, biointeractive cell-ingrowth matrices for vascularized tissue development. FASEB J. 17, 2260–2262 (2003).
Seliktar, D., Zisch, A. H., Lutolf, M. P., Wrana, J. L. & Hubbell, J. A. MMP-2 delicate, VEGF-bearing bioactive hydrogels for promotion of vascular therapeutic. J. Biomed. Mater. Res. A 68, 704–716 (2004).
Zisch, A. H., Schenk, U., Schense, J. C., Sakiyama-Elbert, S. E. & Hubbell, J. A. Covalently conjugated VEGF–fibrin matrices for endothelialization. J. Management. Launch 72, 101–113 (2001).
Moulisová, V. et al. Engineered microenvironments for synergistic VEGF–integrin signalling throughout vascularization. Biomaterials 126, 61–74 (2017).
Lee, T. T. et al. Gentle-triggered in vivo activation of adhesive peptides regulates cell adhesion, irritation and vascularization of biomaterials. Nat. Mater. 14, 352–360 (2015).
Brady, A. C. et al. Proangiogenic hydrogels inside macroporous scaffolds improve islet engraftment in an extrahepatic web site. Tissue Eng. A 19, 2544–2552 (2013).
Liang, J. P., Accolla, R. P., Jiang, Okay., Li, Y. & Stabler, C. L. Managed launch of anti-inflammatory and proangiogenic elements from macroporous scaffolds. Tissue Eng. A 27, 1275–1289 (2021).
Chen, T. T. et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 (2010).
Yin, N. et al. VEGF-conjugated alginate hydrogel immediate angiogenesis and enhance pancreatic islet engraftment and performance in sort 1 diabetes. Mater. Sci. Eng. C 59, 958–964 (2016).
Lee, J., Yang, C., Ahn, S., Choi, Y. & Lee, Okay. Enhanced NO-induced angiogenesis by way of NO/H2S co-delivery from self-assembled nanoparticles. Biomater. Sci. 9, 5150–5159 (2021).
Santulli, G. et al. In vivo properties of the proangiogenic peptide QK. J. Transl. Med. 7, 41 (2009).
D’Andrea, L. D. et al. Concentrating on angiogenesis: structural characterization and organic properties of a de novo engineered VEGF mimicking peptide. Proc. Natl Acad. Sci. USA 102, 14215–14220 (2005). This examine describes the creation of a VEGF mimic with vascularizing properties just like these of the mum or dad protein.
Kumar, V. A. et al. Extremely angiogenic peptide nanofibers. ACS Nano 9, 860–868 (2015). This examine describes the creation of an angiogenic self-assembling peptide–hydrogel with a VEGF mimic within the main sequence.
Kumar, V. A. et al. Remedy of hind limb ischemia utilizing angiogenic peptide nanofibers. Biomaterials 98, 113–119 (2016).
Moore, A. N. et al. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis with out medication, proteins, or cells. Biomaterials 161, 154–163 (2018).
Lopez-Silva, T. L. et al. Chemical performance of multidomain peptide hydrogels governs early host immune response. Biomaterials 231, 119667 (2020).
Carrejo, N. C. et al. Multidomain peptide hydrogel accelerates therapeutic of full-thickness wounds in diabetic mice. ACS Biomater. Sci. Eng. 4, 1386–1396 (2018).
Lopez-Silva, T. L. et al. Self-assembling multidomain peptide hydrogels speed up peripheral nerve regeneration after crush damage. Biomaterials 265, 120401 (2021).
Lai, C. S. E. et al. A mixed conduit-bioactive hydrogel strategy for regeneration of transected sciatic nerves. ACS Appl. Bio Mater. 2022, 4611–4624 (2022).
Howard, D., Buttery, L. D., Shakesheff, Okay. M. & Roberts, S. J.Tissue engineering: methods, stem cells and scaffolds. J. Anat. 213, 66–72 (2008).
Kinstlinger, I. S. & Miller, J. S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 16, 2025–2043 (2016).
Sarker, M. D., Naghieh, S., Sharma, N. Okay. & Chen, X. 3D biofabrication of vascular networks for tissue regeneration: a report on current advances. J. Pharm. Anal. 8, 277–296 (2018).
Devillard, C. D. & Marquette, C. A. Vascular tissue engineering: challenges and necessities for a perfect massive scale blood vessel. Entrance. Bioeng. Biotechnol. 9, 721843 (2021).
Hynes, W. F. et al. Inspecting metastatic conduct inside 3D bioprinted vasculature for the validation of a 3D computational circulate mannequin. Sci. Adv. 6, eabb3308 (2020).
Kinstlinger, I. S. et al. Era of mannequin tissues with dendritic vascular networks by way of sacrificial laser-sintered carbohydrate templates. Nat. Biomed. Eng. 4, 916–932 (2020).
Papaioannou, T. G. & Stefanadis, C. Vascular wall shear stress: primary ideas and strategies. Hell. J. Cardiol. 46, 9–15 (2005).
Yilmaz, B., Al Rashid, A., Mou, Y. A., Evis, Z. & Koç, M. Bioprinting: a evaluate of processes, supplies and functions. Bioprinting 23, e00148 (2021).
Emerson, A. E., McCall, A. B., Brady, S. R., Slaby, E. M. & Weaver, J. D. Hydrogel injection molding to generate advanced cell encapsulation geometries. ACS Biomater. Sci. Eng. 8, 4002–4013 (2022).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with excessive mobile density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019). This examine describes a technique for quickly creating vascular networks between densely packed mobile aggregates, for tissue engineering functions.
Lee, A. et al. 3D bioprinting of collagen to rebuild elements of the human coronary heart. Science 365, 482–487 (2019). Fabricates large-scale tissue engineering scaffolds utilizing extrusion 3D printing.
Grigoryan, B. et al. Multivascular networks and practical intravascular topologies inside biocompatible hydrogels. Science 364, 458–464 (2019). This examine describes the usage of 3D printing to manufacture high-resolution constructions utilizing biocompatible supplies.
Kelly, B. E. et al. Volumetric additive manufacturing by way of tomographic reconstruction. Science 363, 1075–1079 (2019).
Regehly, M. et al. Xolography for linear volumetric 3D printing. Nature 588, 620–624 (2020).
Anandakrishnan, N. et al. Quick stereolithography printing of large-scale biocompatible hydrogel fashions. Adv. Healthc. Mater. 10, 2002103 (2021).
Galarraga, J. H., Kwon, M. Y. & Burdick, J. A. 3D bioprinting by way of an in situ crosslinking method in the direction of engineering cartilage tissue. Sci. Rep. 9, 19987 (2019).
Ouyang, L., Highley, C. B., Solar, W. & Burdick, J. A. A generalizable technique for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv. Mater. 29, 1604983 (2017).
Datta, P., Dey, M., Ataie, Z., Unutmaz, D. & Ozbolat, I. T. 3D bioprinting for reconstituting the most cancers microenvironment. npj Summary. Oncol. 4, 18 (2020).
Ma, X. et al. Deterministically patterned biomimetic human iPSC-derived hepatic mannequin by way of speedy 3D bioprinting. Proc. Natl Acad. Sci. USA 113, 2206–2211 (2016).
Skylar-Scott, M. A., Mueller, J., Visser, C. W. & Lewis, J. A. Voxelated smooth matter by way of multimaterial multinozzle 3D printing. Nature 575, 330–335 (2019).
Shiwarski, D. J., Hudson, A. R., Tashman, J. W. & Feinberg, A. W. Emergence of FRESH 3D printing as a platform for superior tissue biofabrication. APL Bioeng. 5, 010904 (2021).
Gao, G. et al. Building of a novel in vitro atherosclerotic mannequin from geometry-tunable artery equivalents engineered by way of in-bath coaxial cell printing. Adv. Funct. Mater. 31, 2008878 (2021).
Cho, W. W., Ahn, M., Kim, B. S. & Cho, D. W. Blood-lymphatic built-in system with heterogeneous melanoma spheroids by way of in-bath three-dimensional bioprinting for modelling of combinational focused remedy. Adv. Sci. 9, e2202093 (2022).
Kim, B. S. et al. Building of tissue-level cancer-vascular mannequin with high-precision place management by way of in situ 3D cell printing. Small Strategies 5, 2100072 (2021).
Singh, N. Okay. et al. Coaxial cell printing of a human glomerular mannequin: anin vitroglomerular filtration barrier and its pathophysiology. Biofabrication 15, 024101 (2023).
Boularaoui, S., Al Hussein, G., Khan, Okay. A., Christoforou, N. & Stefanini, C. An outline of extrusion-based bioprinting with a concentrate on induced shear stress and its impact on cell viability. Bioprinting 20, e00093 (2020).
Wang, Z. et al. A easy and high-resolution stereolithography-based 3D bioprinting system utilizing seen gentle crosslinkable bioinks. Biofabrication 7, 045009 (2015).
Derakhshanfar, S. et al. 3D bioprinting for biomedical units and tissue engineering: a evaluate of current tendencies and advances. Bioact. Mater. 3, 144–156 (2018).
Hwang, H. H., Zhu, W., Victorine, G., Lawrence, N. & Chen, S. 3D-printing of practical biomedical microdevices by way of light- and extrusion-based approaches. Small Strategies 2, 1700277 (2018).
Ikuta, Okay. & Hirowatari, Okay. Actual three dimensional micro fabrication utilizing stereo lithography and steel molding. In Proc. IEEE Micro Electro Mechanical Programs 42–47 (IEEE, 2002).
Fonseca, A. C. et al. Emulating human tissues and organs: a bioprinting perspective towards personalised drugs. Chem. Rev. 120, 11093–11139 (2020).
Warner, J., Soman, P., Zhu, W., Tom, M. & Chen, S. Design and 3D printing of hydrogel scaffolds with fractal geometries. ACS Biomater. Sci. Eng. 2, 1763–1770 (2016).
Zhu, W. et al. Direct 3D bioprinting of prevascularized tissue constructs with advanced microarchitecture. Biomaterials 124, 106–115 (2017).
Tomov, M. L. et al. A 3D bioprinted in vitro mannequin of pulmonary artery atresia to guage endothelial cell response to microenvironment. Adv. Healthc. Mater. 10, 2100968 (2021).
Bagheri Saed, A. et al. An in vitro examine on the important thing options of poly l-lactic acid/biphasic calcium phosphate scaffolds fabricated by way of DLP 3D printing for bone grafting. Eur. Polym. J. 141, 110057 (2020).
Jiang, P. et al. Grayscale stereolithography of gradient hydrogel with site-selective form deformation. Adv. Mater. Technol. 7, 2101288 (2022).
You, S. et al. Mitigating scattering results in light-based three-dimensional printing utilizing machine studying. J. Manuf. Sci. Eng. 142, 081002 (2020).
Bernal, P. N. et al. Volumetric bioprinting of organoids and optically tuned hydrogels to construct liver-like metabolic biofactories. Adv. Mater. 34, 2110054 (2022).
You, S. et al. Excessive cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 9, eade7923 (2023).
Seymour, A. J., Westerfield, A. D., Cornelius, V. C., Skylar-Scott, M. A. & Heilshorn, S. C. Bioprinted microvasculature: progressing from construction to operate. Biofabrication 14, 022002 (2022). This evaluate offers a complete overview of the challenges and methods surrounding the 3D printing of vasculature.
Urciuolo, A. et al. Hydrogel-in-hydrogel stay bioprinting for steering and management of organoids and organotypic cultures. Nat. Commun. 14, 3128 (2023).
Betz, C., Lenard, A., Belting, H. G. & Affolter, M. Cell behaviors and dynamics throughout angiogenesis. Growth 143, 2249–2260 (2016).
Labarrere, C. A., Dabiri, A. E. & Kassab, G. S. Thrombogenic and inflammatory reactions to biomaterials in medical units. Entrance. Bioeng. Biotechnol. 8, 123 (2020).
Zheng, Y. et al. In vitro microvessels for the examine of angiogenesis and thrombosis. Proc. Natl Acad. Sci. USA 109, 9342–9347 (2012).
Kelly, B. E. et al. Computed axial lithography (CAL): towards single step 3D printing of arbitrary geometries. Preprint at https://arxiv.org/abs/1705.05893 (2017).
Hutson, C. B. et al. Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng. A 17, 1713–1723 (2011).
Ye, W. et al. 3D printing of gelatin methacrylate-based nerve steering conduits with a number of channels. Mater. Des. 192, 108757 (2020).
Zhang, B. et al. Strengths, weaknesses, and functions of computational axial lithography in tissue engineering. Biodes. Manuf. 3, 5–6 (2020).
Hiob, M. A., She, S., Muiznieks, L. D. & Weiss, A. S. Biomaterials and modifications within the improvement of small-diameter vascular grafts. ACS Biomater. Sci. Eng. 3, 712–723 (2017).
Ravi, S. & Chaikof, E. L. Biomaterials for vascular tissue engineering. Regen. Med. 5, 107–120 (2010).
Jang, T. S. et al. 3D printing of hydrogel composite techniques: current advances in know-how for tissue engineering. Int. J. Bioprint 4, 126 (2018).
Akentjew, T. L. et al. Fast fabrication of strengthened and cell-laden vascular grafts structurally impressed by human coronary arteries. Nat. Commun. 10, 3098 (2019).
Pei, B. et al. Fiber-reinforced scaffolds in smooth tissue engineering. Regen. Biomater. 4, 257–268 (2017).
Wang, Z. et al. 3D-printable self-healing and mechanically strengthened hydrogels with host–visitor non-covalent interactions built-in into covalently linked networks. Mater. Horiz. 6, 733–742 (2019).
Zhang, J. et al. 3D printing of silk particle-reinforced chitosan hydrogel constructions and their properties. ACS Biomater. Sci. Eng. 4, 3036–3046 (2018).
Mobile & gene remedy guidances, FDA https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (2024).
LAVIV. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/laviv (2018).
Schurr, M. J. et al. Section I/II scientific analysis of StrataGraft: a constant, pathogen-free human pores and skin substitute. J. Trauma 66, 866–874 (2009).
Grigoryan, B. et al. Growth, characterization, and functions of multi-material stereolithography bioprinting. Sci. Rep. 11, 3171 (2021).
Camasão, D. B. & Mantovani, D. The mechanical characterization of blood vessels and their substitutes within the steady quest for physiological-relevant performances. A crucial evaluate. Mater. Immediately Bio 10, 100106 (2021).
Erbel, R. & Eggebrecht, H. Aortic dimensions and the danger of dissection. Coronary heart 92, 137–142 (2006).
Keelan, J. & Hague, J. P. The position of vascular complexity on optimum junction exponents. Sci. Rep. 11, 5408 (2021).
Sherman, T. F. On connecting massive vessels to small. The which means of Murray’s legislation. J. Gen. Physiol. 78, 431–453 (1981).
Painter, P. R., Edén, P. & Bengtsson, H. U. Pulsatile blood circulate, shear pressure, vitality dissipation and Murray’s legislation. Theor. Biol. Med. Mannequin. 3, 31 (2006).
Morawietz, H. et al. Regulation of the endothelin system by shear stress in human endothelial cells. J. Physiol. 525, 761–770 (2000).
Taylor, D. J. et al. Refining our understanding of the circulate by means of coronary artery branches; revisiting Murray’s legislation in human epicardial coronary arteries. Entrance. Physiol. 13, 871912 (2022).
Adam, J. A. Blood vessel branching: past the usual calculus drawback. Math. Magazine. 84, 196–207 (2011).
Xu, J. & Shi, G. P. Vascular wall extracellular matrix proteins and vascular illnesses. Biochim. Biophys. Acta 1842, 2106–2119 (2014).
Tucker, W. D., Arora, Y. & Mahajan, Okay. Anatomy, blood vessels. in StatPearls [Internet] (StatPearls Publishing, 2023); https://pubmed.ncbi.nlm.nih.gov/29262226/
Russo, T. A., Banuth, A. M. M., Nader, H. B. & Dreyfuss, J. L. Altered shear stress on endothelial cells results in reworking of extracellular matrix and induction of angiogenesis. PLoS ONE 15, e0241040 (2020).
Kubota, Y., Kleinman, H. Okay., Martin, G. R. & Lawley, T. J. Function of laminin and basement membrane within the morphological differentiation of human endothelial cells into capillary-like constructions. J. Cell Biol. 107, 1589–1598 (1988).
Kalebic, T., Garbisa, S., Glaser, B. & Liotta, L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science 221, 281–283 (1983).
Marieb, E. N. Necessities of Human Anatomy & Physiology eleventh edn (Pearson, 2015).
Courtney, J. M. & Sutherland, B. Harnessing the stem cell properties of pericytes to restore the mind. Neural Regen. Res. 15, 1021–1022 (2020).
Daneman, R. & Prat, A. The blood–mind barrier. Chilly Spring Harb. Perspect. Biol. 7, a020412 (2015).
Hanrahan, V. et al. The angiogenic swap for vascular endothelial development issue (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D within the adenoma–carcinoma sequence throughout colorectal most cancers development. J. Pathol. 200, 183–194 (2003).
Holmes, D. I. R. & Zachary, I. The vascular endothelial development issue (VEGF) household: angiogenic elements in well being and illness. Genome Biol. 6, 209 (2005).
Chau, Okay., Hennessy, A. & Makris, A. Placental development issue and pre-eclampsia. J. Hum. Hypertens. 31, 782–786 (2017).
Baldwin, M. E. et al. Vascular endothelial development issue D is dispensable for improvement of the lymphatic system. Mol. Cell. Biol. 25, 2441–2449 (2005).
Zhang, F. et al. VEGF-B is dispensable for blood vessel development however crucial for his or her survival, and VEGF-B focusing on inhibits pathological angiogenesis. Proc. Natl Acad. Sci. USA 106, 6152–6157 (2009).
Achen, M. G. et al. Vascular endothelial development issue D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA 95, 548–553 (1998).
Duffy, A. M., Bouchier-Hayes, D. J. & Harmey, J. H. Vascular endothelial development issue (VEGF) and its position in non-endothelial cells: autocrine signalling by VEGF. in Madame Curie Bioscience Database [Internet] (Landes Bioscience, 2013); https://www.ncbi.nlm.nih.gov/books/NBK6482/
Bates, D. O. Vascular endothelial development elements and vascular permeability. Cardiovasc. Res. 87, 262–271 (2010).
Thurston, G. Complementary actions of VEGF and angiopoietin-1 on blood vessel development and leakage. J. Anat. 200, 575–580 (2002).
Krilleke, D., Ng, Y.-S. E. & Shima, D. T. The heparin-binding area confers various capabilities of VEGF-A in improvement and illness: a construction–operate examine. Biochem. Soc. Trans. 37, 1201–1206 (2009).
Park, J. E., Keller, G. A. & Ferrara, N. The vascular endothelial development issue (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 (1993).
Ruhrberg, C. et al. Spatially restricted patterning cues offered by heparin-binding VEGF-A management blood vessel branching morphogenesis. Genes Dev. 16, 2684–2698 (2002).
Catena, R. et al. Elevated expression of VEGF121/VEGF165–189 ratio ends in a major enhancement of human prostate tumor angiogenesis. Int. J. Most cancers 120, 2096–2109 (2007).
Roskoski, R. VEGF receptor protein–tyrosine kinases: construction and regulation. Biochem. Biophys. Res. Commun. 375, 287–291 (2008).
Takahashi, T., Ueno, H. & Shibuya, M. VEGF prompts protein kinase C-dependent, however Ras-independent Raf–MEK–MAP kinase pathway for DNA synthesis in main endothelial cells. Oncogene 18, 2221–2230 (1999).
Eliceiri, B. P. et al. Selective requirement for Src kinases throughout VEGF-induced angiogenesis and vascular permeability. Mol. Cell 4, 915–924 (1999).
Chen, D. & Simons, M. Rising roles of PLCγ1 in endothelial biology. Sci. Sign. 14, eabc6612 (2021).
Perrin, R. M. et al. Diabetic retinopathy is related to a swap in splicing from anti- to pro-angiogenic isoforms of vascular endothelial development issue. Diabetologia 48, 2422–2427 (2005).