In a current research printed in Nature, Chen et al.1 investigated the potential of RBPJ knock-out to enhance stability and performance of induced regulatory T cells (iTreg cells). This discovering might additional improve the utilization of those cells for adoptive immunotherapy in immune-related illnesses.
Regulatory T cells (Treg cells) play an important function in sustaining immune homeostasis. They’re a subset of CD4-positive T cells which might be chosen as self-reactive clones throughout thymic maturation. The anti-inflammatory operate of Treg cells is ruled by the transcription issue forkhead-box P3 (FOXP3)2 and a thymus-induced epigenetic framework.3 As indispensable regulators of different immune cells, Treg cells guarantee immune tolerance in direction of self and stop autoimmune responses. Past their function in immune regulation, Treg cells additionally exhibit tissue-resident features, migrating into organs to advertise homeostasis and tissue restore upon injury.4
Treg cells possess anti-inflammatory and tissue homeostasis-promoting properties, making them a sexy choice for adoptive cell remedy in sufferers with transplant rejection or graft-versus-host illness. As well as, the potential of Treg cells is investigated in varied autoimmune illnesses, together with psoriasis, systemic lupus erythematosus, rheumatoid arthritis, and kind 1 diabetes. To completely discover the therapeutic potential of Treg cells, it’s important to generate secure Treg cells in adequate numbers. One promising strategy for producing Treg cells is thru ex vivo growth of T cells within the presence of particular cytokines, akin to tumor progress issue β (TGFβ) and interleukin 2 (IL-2). This technique has been proven to induce the differentiation of T cells into useful Treg cells, providing a promising avenue for the event of Treg-cell-based therapies.
Nonetheless, the scientific applicability of those in vitro induced Treg cells (iTreg cells) has been restricted by their suboptimal long-term stability. To handle this problem, Chen et al.1 have recognized a beforehand unknown mechanism that impairs iTreg cell stability and performance. Their findings present new insights into the complicated processes governing iTreg cell operate and stability, and provide an intriguing speculation for the way iTreg cell era could also be improved sooner or later. This analysis has vital implications for the event of efficient Treg-cell-based therapies for autoimmune illnesses. The identification of the transcription issue RBPJ (Recombination Sign Binding Protein For Immunoglobulin Kappa J Area) as a detrimental regulator of iTreg cell stability highlights the significance of epigenetic regulation within the improvement and performance of Treg cells.
Chen et al. utilized CRISPR screens and developed novel methods termed intracellular RNA-seq (icRNA-seq), intracellular chromatin immunoprecipitation adopted by sequencing (inChIP-seq), and intracellular assay for transposase-accessible chromatin utilizing sequencing (inATAC-seq), which permit sequencing of cells stratified by intracellular protein expression. They discovered that FOXP3 ranges, which they used as a proxy for iTreg stability, have been related to information RNAs towards RBPJ. This indicated that RBPJ negatively impacts iTreg cell stability. RBPJ knock-out led to elevated expression of Treg id genes in addition to immunosuppressive genes in iTreg cells. In keeping with this, Chen et al. noticed that iTreg cells might suppress different T cells extra potently after RBPJ knock-out. The results of RBPJ deficiency on iTreg cell stability might even be proven underneath pro-inflammatory tradition situations involving a number of T cell receptor stimulations and publicity to tumor necrosis issue (TNF).
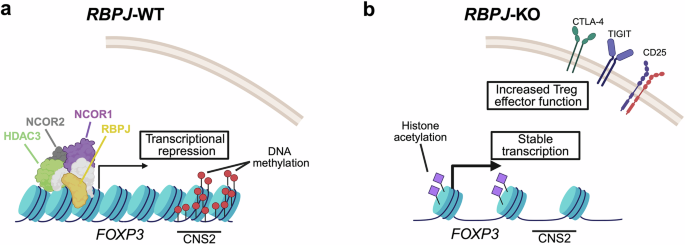
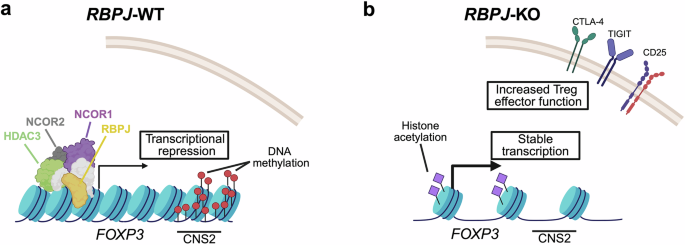
The RBPJ protein participates in Notch sign transduction. If not activated, nevertheless, RBPJ types a transcriptional repressor complicated with different proteins like nuclear receptor co-repressor 1/2 (NCOR1/2) and the histone deacetylase 3 (HDAC3). Apparently, Chen et al. discovered proof that RBPJ-mediated results on FOXP3 ranges have been impartial from Notch signaling and have been particular to the iTreg cell induction context. Mechanistically, Chen et al. established that RBPJ can straight bind to the FOXP3 promoter. Moreover, they supply proof that the meeting of the repressor complicated, together with RBPJ, NCOR1/2, and HDAC3, is necessary for RBPJ-mediated inhibition of FOXP3 expression. HDAC3 seems to be of specific significance on this context, as HDAC3 knock-out reverted an noticed impairment in iTreg cell differentiation upon RBPJ overexpression. In keeping with this, Chen et al. spotlight a number of epigenetic traits which might be linked to RBPJ-mediated suppression of FOXP3. As an illustration, they report that RBPJ knock-out results in a discount in DNA methylation at an necessary intronic FOXP3 enhancer referred to as the “conserved non-coding sequence 2” (CNS2). Moreover, RBPJ knock-out elevated histone 3 acetylation and chromatin accessibility across the FOXP3 promoter, each of which symbolize epigenetic traits related to elevated transcription (Fig. 1).
Mannequin of how RBPJ knock-out can assist within the era of secure iTreg cells. a Scenario in wild-type iTreg cells. The RBPJ protein types a repressive complicated with different proteins, together with NCOR1, NCOR2, and HDAC3. The enhancer area CNS2 within the first intron of the FOXP3 gene is methylated, and FOXP3 is just not stably expressed or expression is misplaced throughout growth. b Scenario in iTreg cells upon RBPJ knock-out. Chromatin accessibility and histone acetylation across the FOXP3 promoter are elevated. CNS2 is demethylated. FOXP3 is constantly transcribed, resulting in a secure Treg cell id. Treg cell effector genes are extra strongly expressed, resulting in enhanced Treg cell operate. CNS conserved non-coding sequence, CTLA4 cytotoxic T-lymphocyte related protein 4, FOXP3 forkhead-box P3, HDAC3 histone deacetylase 3, KO knock-out, NCOR nuclear co-receptor co-repressor, RBPJ recombination sign binding protein for immunoglobulin kappa J area, TIGIT T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif area, WT wild-type. The determine was generated utilizing BioRender.com
Lastly, Chen et al. explored the significance of RBPJ in vivo by testing iTreg cells with or with out RBPJ knock-out in a graft-versus-host illness mannequin in humanized mice. RBPJ knock-out rendered iTreg cells extra secure on this setting. Moreover, RPBJ knock-out in iTreg cells considerably improved the survival of affected mice. Survival was even akin to the degrees noticed with “pure” Treg cells (i.e., purified CD4+CD127lowCD25excessive T cells) that didn’t need to be generated from different T cell varieties.
Altogether, these findings set up that RBPJ impairs the steadiness and performance of Treg cells which might be induced ex vivo. Its perturbation might thus be an acceptable technique to enhance therapies primarily based on iTreg cells, however scientific functions will probably require extra intensive preclinical experimentation. Up to now, in vivo knowledge are solely out there for a graft-versus-host illness setting wherein iTreg cells have been administered at illness onset. Experiments exploring different immune-related illnesses with iTreg cell administration at later time factors (i.e., at full-blown illness) will increase confidence within the therapeutic potential of iTreg cells with RBPJ knock-out. As well as, knowledge from human sufferers might want to present that such cells are efficient in a real-world scientific setting. There’s a caveat associated to the truth that Rbpj knock-out in mice was reported to have the other impact to what Chen et al. noticed in human cells.5 Though Chen et al. talk about a possible cause for this, the discrepancy might point out a but unknown distinction between people and mice with respect to RBPJ and iTreg cells. Preclinical research using non-humanized mice thus must train substantial warning with a purpose to keep away from conclusions which might be reverse to what will probably be noticed in human sufferers.
In conclusion, the outcomes offered by Chen et al. spotlight a promising technique for the development of iTreg cell therapies. Moreover, their CRISPR screens yielded a wealthy knowledge set of potential FOXP3 regulators which will result in much more discoveries of how iTreg cell stability and performance will be improved. Thus, Chen et al. have made a worthwhile contribution to potential therapies of immune-related illnesses utilizing iTreg cell adoptive switch methods.