Zero level cost
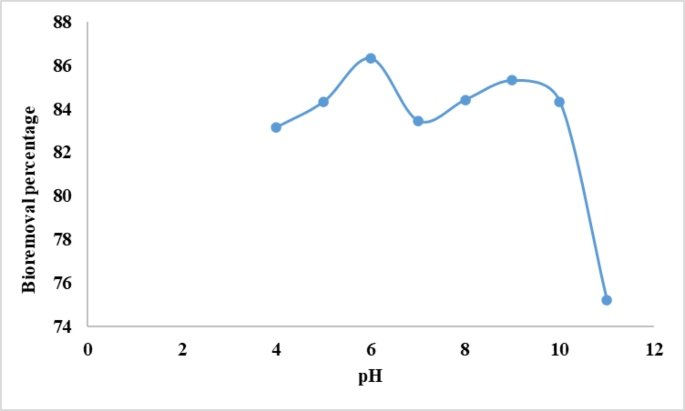
The pHZPC worth is obtained from the plot of pHi vs pHe by batch equilibration. The biosorbent’s zero level cost (pHₚzc) was decided to be 9, suggesting that the floor has a web constructive cost at pH values beneath 9 and a web detrimental cost above this pH. Curiously, it was discovered that the optimum pH for crystal violet biosorption was 6 that may be a pH decrease than the pHₚzc. Though this will seem contradictory at first, there are a variety of the reason why this conduct happens. The biosorbent’s floor has a small constructive cost at pH 6, which usually prevents the cationic dye from adhering due to electrostatic repulsion. Nonetheless, biosorption will not be ruled solely by electrostatic pressure. Regardless of floor cost results, useful teams can nonetheless take part in hydrophobic interactions, hydrogen bonding, and π–π interactions with the fragrant rings of crystal violet so selling adsorption34. Moreover, at elevated pH ranges (> pHₚzc), whereas the floor acquires a detrimental cost and electrostatic attraction is enhanced, components equivalent to precipitation, competitors with OH− ions, or dye aggregation could diminish biosorption effectivity35. Thus, pH 6 seemingly represents a stability between adequate solubility of the dye and energetic useful group availability on the biosorbent floor, resulting in the noticed optimum biosorption. Comparable outcomes have been documented in earlier analysis, the place optimum dye absorption proceeded beneath the biosorbent’s pHₚzc, suggesting the participation of non-electrostatic interactions36.
Preliminary pH
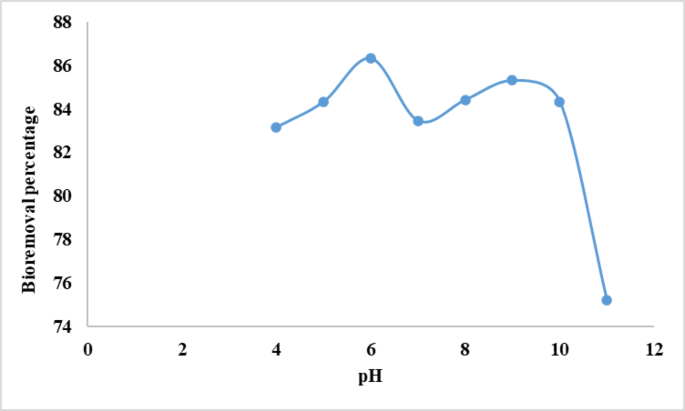
The pH is a key variable in adsorption analysis as a result of it influences adsorption effectivity in addition to coloration and solubility of dyes in options. It influences the dye’s chemical traits and the energetic websites on the biosorbent floor37. The impact of pH on the adsorption of crystal violet dye by alginate beads was decided at completely different pH ranges (4–11). The portions of electrostatic costs that the ionized dye molecules ship will rely on the pH of the medium. In options of assorted pH, useful teams on each the adsorbent and adsorbate might be protonated or deprotonated to supply distinct floor costs, inflicting electrostatic attraction or repulsion38. The variation within the proportion of CV elimination with pH is proven in Fig. 1. Determine 1 exhibits that dye elimination will increase barely with growing pH as much as 6.0, then drops, concluding that pH 6 is the optimum for CV biosorption from aquatic options. At decrease pH, protons could compete with positively charged CV dye molecules for adsorption websites, lowering dye absorption and adsorptive potential. As pH elevated to six, CV adsorption onto alginate beads elevated because of dye cationic species electrostatically interacting with the negatively charged floor of alginate beads39. Additional pH improve triggered many hydroxyl ions to attach on to positively charged dye molecules, limiting their adsorption onto adsorbent websites40. In accordance with our outcomes the researchers reported that there’s a important relationship between the pH and the dye elimination effectivity, and it has been said that the perfect pH vary for dye elimination is positioned inside 6 and 10 vary41. In keeping with the present data, El-Naggar et al. 42 confirmed that the utmost biosorption of congo Crimson on Ulva lactuca from aquatic options was reached at pH 6. Furthermore, Onuk et al.43 reported that the optimum biosorption of MB and CV from aquatic options was achieved at pH 7.
Incubation time
Essentially the most important design parameter affecting adsorption course of efficiency is adsorbate-adsorbent contact time. Within the present examine, crystal violet dye bioremoval by alginate beads was evaluated at completely different time intervals. The incubation time impact on the capability of CV biosorption onto alginate beads is introduced in Fig. 2A. The preliminary biosorption price was appreciable, however it steadily decreased till it reached equilibrium at 7 h, it may be attributed to the lower within the thickness of the diffusion layer surrounding the alginate particles44. Furthermore, different mechanisms like binding web site saturation, complexity, and microprecipitation could happen through the slower section of biosorption45. The outcomes reveal that there’s a sturdy electrostatic pressure of attraction in addition to a excessive affinity between the useful teams which might be current on the alginate beads and the CV dye46.
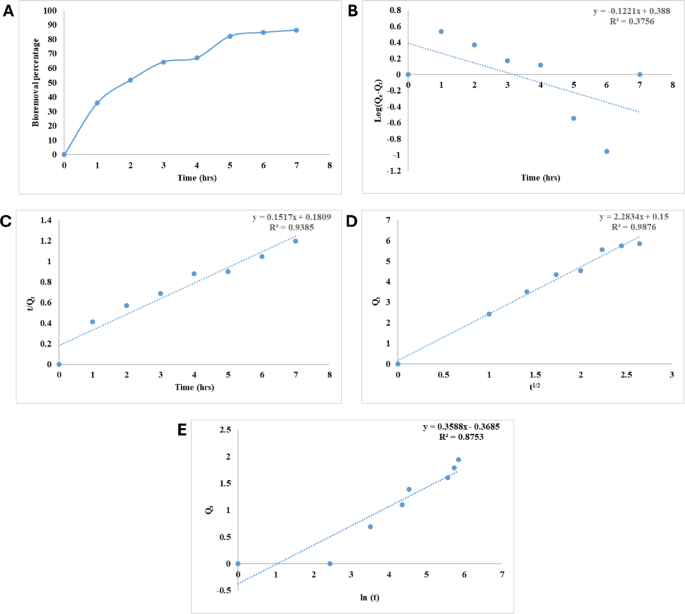
(A) Impact of incubation time on bioremoval effectivity of CV from water by alginate beads on the optimum circumstances temperature (35 °C), dosage of alginate biosorbant (50 mg), CV dye focus of 10 mg L−1, (B) Pseudo-first order kinetic mannequin for CV bioremoval on alginate beads, (C) Pseudo-second order kinetic mannequin for CV bioremoval on alginate beads, (D) Intraparticle diffusion mannequin for CV bioremoval on alginate beads and (E) Elovich kinetic mannequin for CV bioremoval on alginate beads.
So as to decide the kinetic conduct of CV biosortion by alginate beads, the experimental information have been analyzed by the pseudo-first order, pseudo-second order and intraparticle diffusion kinetic fashions. Desk 1 exhibits the parameters and correlation coefficients (R2) for pseudo-first order, pseudo- second order and and intraparticle diffusion fashions. pseudo-first order kinetic mannequin information (Fig. 2B) didn’t match the current outcomes because of the nice distinction between the theoritical Qe (1.474) and the experimental worth (5.884) in addition to the worth of R2 (0.376) which was near zero. Furthermore, The pseudo-second order mannequin offered a extra passable rationalization for the experimental findings (Fig. 2C), and it additionally had a fantastic correlation coefficient (0.939) which was discovered nearer to unity. As well as, the experimental Qe values (5.884) and the computed Qe values (6.592) have been suitable. Therefore, the outcomes level to a course of referred to as chemisorption adsorption47. Adsorption happens in a number of steps, together with the Shifting of CV molecules from the liquid to the alginate beads’ floor and the switch of CV molecules from the floor of the beads into the pores of the alginate48.
The biosorption of crystal violet onto alginate beads within the current examine reached equilibrium at 7 h and adopted pseudo-second-order kinetics, indicating that chemisorption was the dominant mechanism. These findings are in keeping with a number of earlier research. As an example, Mittal et al.49 noticed comparable equilibrium occasions (6–8 h) for crystal violet adsorption onto backside ash and de-oiled soya, with information becoming effectively to a pseudo-second-order mannequin. Likewise, Ismail et al.50 reported most uptake round 7 h utilizing Euphorbia antiquorum-activated carbon. In distinction, different research have proven considerably completely different outcomes. Hameed and Ahmad51 reported a a lot quicker equilibrium time of two h utilizing bamboo-based activated carbon, attributed to the excessive floor space and porosity of the fabric. Annadurai et al.52 discovered that chitin might take away crystal violet inside simply 1 h. These discrepancies spotlight the affect of biosorbent kind, floor traits, and binding web site accessibility on adsorption conduct.
Intraparticle diffusion mannequin of Weber and Morris is often utilized as a way to make clear the adsorption mechanism course of that’s concerned53. When plotting Qt towards t1/2, Weber and Morris fashions predict {that a} straight line could be produced. This is a sign that the adsorption course of is generally managed by intraparticle diffusion, the straight line is anticipated. Movie diffusion or pore diffusion are two attainable examples of the rate-limiting steps54. By decoding Qt versus t1/2, (Fig. 2D) a linear kind was obtained, which signifies the incidence of intraparticle diffusion55. Discovering the slope of the linear part of the kinetics profile yielded the worth of Okint, which stands for the intraparticle diffusion fixed, which is 2.283, whereas Ci was obtained from the intercept and its worth (0.15 mg g−1), gives border thickness data layers as reported by Saad et al.56.
Elovich’s mannequin of activated chemisorption is extraordinarily outstanding. Determine 2E depicts the Elovich mannequin for CV adsorption on alginate beads. From plot intercept and slope, a and b values have been computed and displayed in Desk 1. The correlation coefficient (R2 = 0.875) was decrease than pseudo-second-order mannequin.
Biosorbent dosage
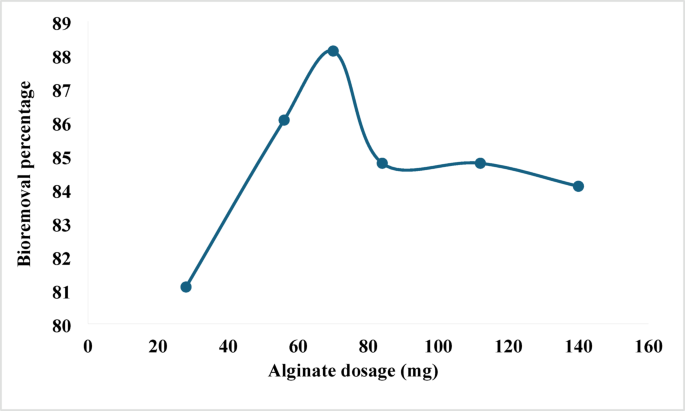
An extra important variable in figuring out the system’s sorbent-sorbate equilibrium for environment friendly pollutant dye elimination is the preliminary adsorbent focus. For the simplest interactions between the sorbate molecules and the sorbent’s adsorption websites in resolution, it’s essential to make use of the suitable quantity of sorbent57. The impact of various calcium alginate beads dosages (28, 56, 70, 84, 112 and 140 mg/ 25 mL) on the biosorption of CV dye from the answer was investigated (Fig. 3). The outcomes reveal that the elimination of dye elevated with growing weight of alginate beads as much as 70 mg due to the rise in accessible energetic adsorption websites quantity. The utmost dye elimination effectivity (88.1%) was obtained by 70 mg of alginate beads. Because of particle aggregation58, or inadequate CV within the resolution59, growing alginate dosage didn’t promote increments in dye elimination. Furthermore, increased dose of biosorbent could end in formation of agglomerates, which in flip reduces the efficient floor space that dye molecules have entry to60.
Within the present examine, the biosorption capability of alginate beads for crystal violet elevated with growing biosorbent dose, reaching optimum elimination at 70 mg. This development is in keeping with earlier analysis, the place growing biosorbent dosage enhanced dye elimination because of the larger availability of energetic binding websites. As an example, Mittal et al.61 reported an analogous constructive correlation when utilizing de-oiled soya and backside ash for crystal violet elimination, observing improved adsorption effectivity with growing sorbent mass as much as a threshold. Likewise, Annadurai et al.52 discovered that increased doses of chitin considerably elevated dye uptake, seemingly because of lowered competitors amongst dye molecules for sorption websites.
Nonetheless, some research have famous a plateau or perhaps a decline in adsorption effectivity at increased doses. Hameed and Ahmad51 utilizing bamboo-based activated carbon, noticed that after a sure level, additional will increase in sorbent mass didn’t considerably enhance elimination effectivity, presumably because of particle aggregation, which reduces the efficient floor space. Equally, Ismail et al.50 discovered that extra biomass would possibly result in web site overlapping or blockage, decreasing the obvious adsorption per unit mass.
These findings counsel that whereas growing biosorbent dose typically enhances elimination effectivity, there exists an optimum dosage such because the 70 mg discovered on this examine past which the biosorption could plateau or diminish, relying on the biosorbent’s construction and interplay dynamics with the dye.
Crystal violet preliminary focus
The preliminary focus of the dye can be one other important issue that has the potential to affect the adsorption course of. Dye concentrations (2–20 mg L−1) various impression on CV biosorption at 35 °C, and the optimum circumstances of pH (6.0), dosage of alginate biosorbant (70 mg), and phone time (5 h) is illustrated in Fig. 4A. Outcomes demonstrated that as dye focus elevated, the efficacy of alginate beads in eradicating CV pollutant dye declined (Fig. 4A). The utmost bioremoval proportion of CV dye (93.917%) was attributed to the bottom dye focus (2 mg L−1). As soon as the dye focus rises to 10 mg L−1 the CV bioremoval proportion decreased dramatically to 62.58% indicating overload of the alginate beads energetic binding websites. Biosorption experimental information are normally approximated as equilibrium isotherms, which give essential parameters for mechanism prediction and course of optimization62.
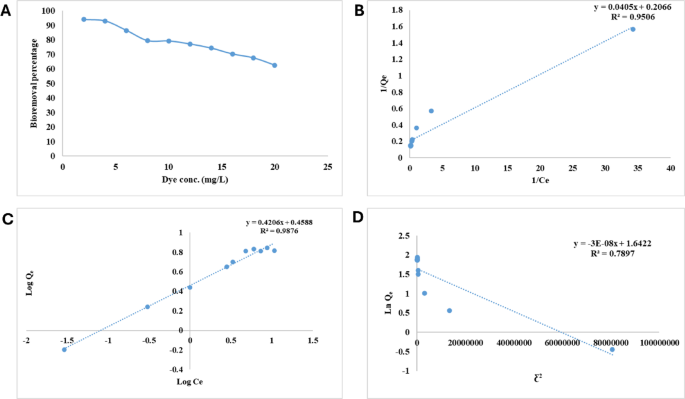
(A) Impact of preliminary CV focus on bioremoval effectivity of CV from water by alginate beads on the optimum circumstances of pH (6.0), temperature (35 °C), dosage of alginate biosorbant (70 mg), and phone time (5 h), (B) Langmuir isotherm for CV bioremoval on alginate beads, (C) Freundlich isotherm for CV bioremoval on alginate beads and (D) Dubinin–Radushkevich isotherm for CV bioremoval on alginate beads.
Within the current examine, the biosorption effectivity of crystal violet by alginate beads decreased with growing preliminary dye focus. Comparable observations have been reported by Chandra et al.49 who discovered that growing the focus of crystal violet led to lowered elimination effectivity when utilizing waste supplies equivalent to de-oiled soya and backside ash. Likewise, Annadurai et al.52 reported that increased concentrations of methylene blue and reactive dyes decreased biosorption by chitin, suggesting that at elevated concentrations, dye molecules compete extra intensely for restricted binding websites. Then again, some research have reported a unique development. For instance, Hameed and Ahmad51 noticed that the adsorption capability (mg g−1) of bamboo-based activated carbon elevated with rising dye focus, though the share elimination decreased, indicating that complete uptake should enhance regardless of decrease effectivity. Equally, Ismail et al.50 discovered that increased preliminary dye concentrations offered a larger driving pressure for mass switch, which enhanced the biosorption price within the early phases. These discrepancies spotlight the significance of distinguishing between proportion elimination and adsorption capability, they usually underline the function of biosorbent floor traits and saturation kinetics.
The Langmuir, Freundlich, and Doubinin–Radushkevich isotherms fashions have been utilized as a way to accomplish the analysis of the biosorption of CV onto alginate beads. Because of its ease of use and excessive diploma of concordance with the experimental information, Langmuir mannequin is taken into account to be some of the prevalent and generally utilized fashions for equilibrium information63. The Langmuir isotherm mannequin assumes biosorption happens in monolayers at homogeneous biosorbent websites64. The outcomes that have been utilized to the Langmuir isotherm mannequin didn’t come near matching the outcomes of the experiments. Based mostly on the plot of 1/Qe vs 1/Ce, which might be present in Fig. 4B, the values of OkL, Qm, and R2 are 5.101 mL mg−1, 4.08 mg g−1, and 0.951, respectively (Desk 2).
The dimensionless fixed RL can be utilized to judge Langmuir biosorption response processes:
$${textual content{R}}_{{textual content{L}}} = {1}/left( {{1} + {textual content{Ok}}_{{textual content{L}}} {textual content{C}}_{{textual content{o}}} } proper)$$
(10)
RL values point out bisorption course of favorability (0 L L > 1), linearity (RL = 1), or irreversibility (RL = 0)37. The worth of the RL fixed for the biosorption of CV by alginate beads was 0.871, which signifies that the biosorption course of is favorable.
For the adsorption of CV dye on alginate beads, the Freundlich isotherm demonstrated that the plot of log Qe vs log Ce ends in a straight line with a slope of 1/n and an intercept of ln OkF (Fig. 4C). The values of Freundlich fixed (OkF), correlation coefficient (R2) and n have been 1.582 mg g−1, 0.988 and a couple of.378, respectively (Desk 2). The worth of n lower than 10 revealing a positive adsorption65. Sum of squares Error was calculated in line with the experimental information and the calculated information, giving 0.501, whereas Root Imply Sq. Error was 0.708. as well as, Akaike criterion data was discovered to be 2.473. Because the adsorption capability is proportional to the worth of OkF as reported by Ibrahim et al.66 so, OkF worth of CV biosorption on alginate beads signifies excessive adsorption capability. In accordance with the findings that we obtained; the method is in keeping with the Freundlich isotherm mannequin. Moreover, alginate beads floor is regarded to be heterogeneous, and the CV is organized in multilayers on the floor of the alginate beads32.
The Dubinin–Radushkevich mannequin (Fig. 4D) describes the Gaussian vitality distribution adsorption mechanism on a heterogeneous floor67 and is broader than is the Langmuir mannequin. Bodily adsorption happens when E is beneath 8 kJ mol–1. Then again, ion trade takes place when E ranges from 8 to16 kJ mol–168. From Desk 2, it’s apparent that vitality worth was greater than 16 so it’s concluded that the impact of chemical adsorption will play a dominating function within the adsorption of CV onto alginate beads. A comparative desk (Desk 3) of CV biosorption of biosorbents is offered69,70,71.
Empirical Redlich–Peterson isotherm mannequin has three parameters. It represents adsorption equilibrium throughout a variety of adsorbate focus in homogenous or heterogeneous programs by combining Langmuir and Freundlich Equations 72. Desk 3 shows the estimated parameters (KR, Redlich-Peterson isotherm fixed (Lg − 1), aR, and bR) utilizing the three-parameter isotherm mannequin to find out the best correlation coefficient. R2 worth of unity was obtained at OkR of two.874 Lg−1 for 8 mg L−1 to twenty mg L−1 crystal violet preliminary focus, demonstrating the benefit of adsorption because the preliminary CV focus rises. Redlich–Peterson approaches the Freundlich isotherm mannequin as bR tends to be lower than 1 supporting multilayer adsorption73. Concluding that, probably the most bR values have been lower than 1 (Desk 4) counsel that this Freundlich mannequin greatest describes CV adsorption on alginate beads.
Alginate beads characterization earlier than and after CV biosorption
FTIR spectroscopy
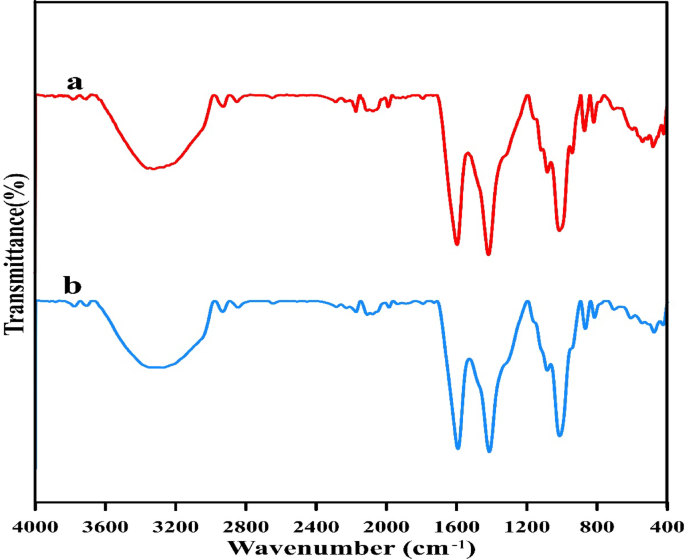
The FTIR spectrums of untreated alginate beads and alginate beads loaded with CV pollutant dye have been analyzed (Fig. 5) to find adjustments brought on by CV-alginate beads useful group interactions throughout biosorption. FTIR evaluation of management alginate beads present completely different adsorption peaks at 3326, 2931, 2851, 1595, 1417, 1116, 1081, 1013, 940, 870, 817, 597, 539, 510, 580 and 419 cm−1 which have been shifted to 3274, 2933, 2845, 1592, 1414, 1084, 1013, 867, 815, 703, 607, 543, 475 and 424 cm−1 after biosorption course of. A powerful absorption band at 3326 cm−1 is noticed within the Fourier rework infrared (FTIR) spectrum of management alginate beads powder, which signifies the presence of hydroxyl teams as anticipated74. Aside from fragrant and/or vinylic C–H stretching, spectral band at 2931 cm−1 might be ascribed to aliphatic –CH stretching and symmetric and asymmetrical (C–H)CH2 stretching, (CH)-anomer stretching75. The absorbance at 2851 cm−1 represents –CH3 teams76. The spectrum band at 1995 cm−1 exhibits the stretching vibrations of carboxylate anions (COO–), which might be both uneven or symmetric75. Spectral band that positioned 1417 cm−1 is expounded to –N–H bending77. The sodium alginate polysaccharide construction can be characterised by the opposite peaks that come up within the area between 1190 and 900 cm−1. The [C–O] and [C–C] stretching vibrations are linked to those peaks78.
The absorption peak at 870 cm−1 wave quantity is the attribute absorption peak of CO3 vibrations of calcite79. The spectral band at 940 cm−1 is attributed to O–H bending out of airplane80. Alginate’s major parts, guluronic and mannuronic acids, are revealed by a spectral peak at 817 cm−1, giving it a definite spectral signature that units it aside from different polysaccharides81. Furthermore, Khattar et al.82 steered that the existence of a number of peaks lower than 1000 cm−1 could also be in line with varied seen bands and/or to the existence of some linkages amongst monosaccharides.
There’s a shift within the wave variety of the Peaks that happens after CV biosorption, both upwards or downwards. The hydroxyl group absorption peak at 3326 cm−1 was shifted to 3274 cm−1 which is presumably because of the interplay of O–H group with COO − Na + to kind carboxymethyl teams83. Furthermore, the peaks 2931, 2851, 1595 and 1417 have been shifted to 2933, 2845, 1592 and 1414 cm−1. Additionally a spectral band of 1081, 870 and 817 cm−1 have been shifted to 1084, 867 and 815 cm−1. A brand new spectral band appeared in alginate beads loaded with CV at 703 cm−1 which can be attributed to C–C1 stretching84. Furthermore, the spectral band at 597 cm−1 was shifted to 607 cm−1. The height at 539 cm−1 was shifted to 543 cm−1 indicating uneven deformation vibration of P=O in PO43,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85. The spectral band at 480 and 419 cm−1 have been shifted to 475 and 424 cm−1, respectively. Because of the truth that there was a slight distinction within the variety of peaks that occurred after and earlier than the CV bioremoval, it was assumed that the dye that was current within the calcium alginate beads was interacting with the energetic useful teams86. In conclusion, the FITR evaluation demonstrated that the carboxylic, methyl, and hydroxyl teams have been the first teams that have been engaged within the CV biosorption course of. For the CV binding, the carboxylic useful group is liable for offering the vast majority of the biosorption websites87.
Scan electron microscopic imaging
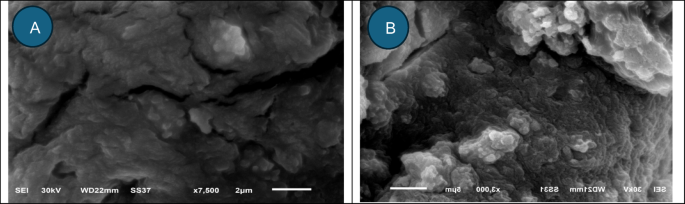
Alginate beads is a superb materials for CV bioremoval, was examined through SEM. Alginate beads earlier than and after loading with crystal violet are introduced in Fig. 6a,b respectively. In keeping with current information, Malakar et al.88 revealed that alginate beads possess a spherical morphology with tough exterior surfaces, and that the spherical configuration is often compromised upon drying. The cross-sectional SEM photos revealed many closed holes of assorted widths upon loading with CV.