Moral Assertion
The protocols used for the Pcsk9-EC animal research, MM-Cxcl-EC animal research, and LNP biodistribution research have been reviewed and acquired moral approval from the Pharma Fashions (Marlborough, MA), MLM Medical Labs (Minneapolis, MN), and Biomere Biomedical Analysis Fashions, Inc. (Worcester, MA) Institutional Animal Care and Use Committees (IACUC), respectively. Animal research have been carried out by Pharma Fashions, MLM Medical Labs, or Biomere within the presence of an attending veterinarian on the facility, as crucial, and all animal welfare considerations have been addressed and documented. Intercourse was not thought of for the research design, as solely feminine mice have been used. Feminine mice have been used for housing functions. Due to this fact, disaggregated intercourse info was not crucial.
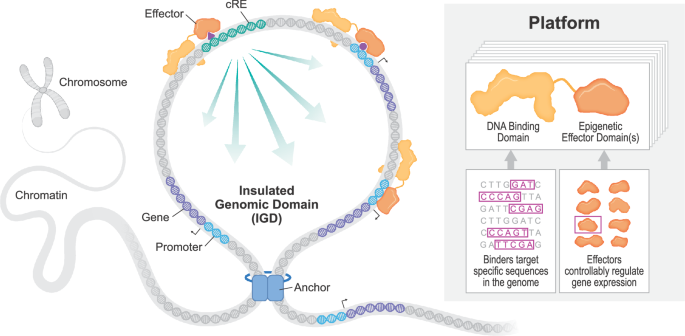
Design and manufacturing of epigenetic controller mRNA
Controllers have been designed to focus on particular DNA sequences inside genomic regulatory parts of curiosity in-house. Synthesis of the coding sequence of every controller was carried out by ATUM (Newark, CA) and subcloned into the suitable in vitro translation (IVT)- succesful spine vector. Plasmids have been scaled up, and sequences have been confirmed by Sanger sequencing previous to IVT. For mRNA manufacturing, plasmids have been linearized with SapI and purified utilizing a phenol:choloroform extraction. The linearized plasmid was then added to an in vitro transcription (IVT) response utilizing a T7 RNA polymerase, unmodified NTPs, Hepes buffer, magnesium chloride, DTT, spermidine, RNase inhibitors, and pyrophosphatase at proprietary concentrations. This response was incubated at 41 °C for two hours. This crude IVT combination was then handled with DNase to digest the linearized plasmid, and the ensuing mRNA was capped in an enzymatic response comprised of Hepes buffer, potassium chloride, DTT, GTP, SAM, RNase inhibitors, guanylyltransferase and 2-O-methyltransferase at proprietary concentrations at 37 oC for 1 h. This combination was then purified utilizing OligodT chromatography and eluted in molecular biology-grade water.
Preparation of LNPs for in-vitro transfection
Lipid nanoparticles have been produced for in vitro transfection by way of microfluidic mixing utilizing the NanoAssemblrTM SparkTM (Precision NanoSystems). mRNA encoding an Epigenomic Controller (EC) at 0.4 mg/mL in both 50 mM citric acid buffer, pH 4.5, or 50 mM malic acid buffer, pH 4.5, was blended with an ethanolic lipid part at Circulate Price Ratio = 3:1 (aqueous: natural). The ethanol part contained both MC3 ((6Z,9Z,28Z,31Z)-heptatriacont-6,9,28,31-tetraene-19-yl 4-(dimethylamino)butanoate) or SS-OP (4-[[(9Z)-1-oxo-9-octadecen-1-yl]oxy]-benzeneacetic acid, 1,1′-[dithiobis(2,1-ethanediyl-1,4-piperidinediyl-2,1-ethanediyl)] ester) as the principle ionizable lipid, ldl cholesterol, DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and DMG-PEG2000 (1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000) at 45:44:9:2 mole ratio. The ionizable-lipid-to-RNA mass ratios have been 6:1 and 10:1 for MC3 and SSOP, respectively.
LNPs have been collected in 3 volumes of 1X phosphate-buffered saline, pH 7.4 post-mixing to dilute ethanol content material and permit for correct formation of the nanoparticles. The ultimate mRNA focus was adjusted with a cell tradition medium instantly previous to transfection.
Preparation of LNPs for in vivo dosing in mice
Lipid nanoparticles have been produced for dosing in mice by way of microfluidic mixing utilizing the NanoAssemblrTM IgniteTM (Precision NanoSystems). mRNA encoding a reporter protein (Cre Recombinase) or an Epigenomic Controller (EC) at 0.25-1 mg/mL in 50 mM citrate buffer, pH 4.5 was blended with an ethanolic lipid part at Circulate Price Ratio = 2:1 (aqueous: natural).
For Pcsk9 research, the ethanol part contained MC3 as the principle ionizable lipid, ldl cholesterol, DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), and DMG-PEG2000 (1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000) at 50:38.5:10:1.5 mole ratio. The MC3-to-RNA mass ratio used was 6:1.
For biodistribution and Cxcl research, the ethanol part contained DOTAP as principal cationic lipid (1,2-dioleoyl-3-trimethylammonium-propane), ldl cholesterol, DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and DMG-PEG2000 (1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000) at 45:45:9:1 mole ratio. The DOTAP-to-RNA mass ratio used was 6:1.
LNPs have been dialyzed post-mixing towards storage buffer (pH 7, 8–10% sucrose) for ethanol removing, following focus by tangential circulate filtration and sterile filtration previous to storage at −80 °C. LNP diameter ranged from 60–100 nm as measured by dynamic gentle scattering (Wyatt Mobius). RNA encapsulation was decided by way of RiboGreen assay to be above 90% for all LNPs produced.
In vitro transcriptional and protein read-outs
All cell traces, together with NIH3T3 (ATCC #CRL-1658), IMR90 (ATCC #CCL-186), Ok-562 (ATCC #CCL-243), Hep3B (ATCC #HB-8064), and Hepa1-6 (ATCC #CRL-1830), have been sourced from and authenticated by the ATCC and grown in a humidified 37 oC incubator with 5% CO2. Cultures have been routinely examined for mycoplasma contamination by PCR. Cells weren’t in any other case authenticated. Cells have been transfected with LNPs containing controller mRNA, which was added to the media at a closing focus of 1 µg/mL of mRNA. After a 48 h incubation, IL1-alpha (PCH0015, Life Applied sciences) was added at 10 ng/mL closing focus, and the samples have been incubated for 1 h. For protein, supernatant was collected after a 1 h incubation with IL1-Alpha, and cytokines have been measured utilizing the ProcartaPlex 7-plex package (Thermo Fisher Scientific, Cat# PPX-07-MXT2AW2, Lot# 333491-000/331205-000). Protein ranges have been decided utilizing the Luminex FlexMAP 3D Multiplexing Microplate reader (Thermo Fisher Scientific). After 1 h incubation with IL1-Alpha, RNA was remoted utilizing an RNA extraction package (Macherey-Nagel Inc., Cat# 740466.4).
Quantitative PCR
RNA samples have been retrotranscribed to cDNA utilizing LunaScript RT SuperMix Equipment following the producer’s protocol (New England Biolabs, Cat# E3010) and analyzed by quantitative PCR utilizing the person particular Taqman primer/probe units (Supplementary Information 1) with the Taqman Quick Superior Grasp Combine (Thermo Fisher Scientific, Cat# 4444558).
Protein Immunodetection
Complete-cell lysates (WCEs) have been ready by means of lysing cell pellets for 3 min in 1X protein pattern loading buffer (LICORbio, Cat# 928-40004) supplemented with 10 mM β-mercaptoethanol and Halt Protease Inhibitor Cocktail (ThermoFisher Scientific, Cat# 78429) then subjected to sonication on a PIXUL Multi-Pattern Sonicator (Lively Motif, Cat #53130) in accordance with the producer’s beneficial settings. WCEs have been clarified and run on a Jess Automated Western System (bio-techne/protein easy, Cat # 004-650) utilizing the 12-230 kDa Separation Module (bio-techne/protein easy, Cat # SM-W001) in accordance with producer’s beneficial settings and using an HA antibody (1:20 dilution, Cell Signaling Know-how, Cat # CST2367S)and a CTCF loading management antibody (1:200 dilution, Cell Signaling Know-how, Cat # CST3418S).
Neutrophil migration assay
IMR-90 cells have been cultured and plated in EMEM (ATCC, Cat# 30-2003) at 100k cells/effectively in 6 effectively plates. Full media was made with 10% FBS (VWR, Cat# 97068-085). IMR-90 cells have been transfected by including CXCL-EC to the media at a closing focus vary of 1 μg/mL to 0.0008 μg/mL of mRNA formulated in SSOP lipid combine. Cells have been incubated for twenty-four h with media containing LNPs. After 24 h, IL1-alpha (Life Applied sciences, Cat# PCH0015) was added at 10 ng/mL closing focus and incubated for 32 h. After incubation with IL1-alpha, the cell supernatant was collected used to conduct a neutrophil migration assay utilizing IL1-alpha handled supernatant as a management to which the take a look at teams have been in comparison with a decrease chamber, and neutrophils from six completely different human plasma donors have been added individually to an higher chamber. After 1 h, the variety of neutrophils have been quantified on the decrease chamber to evaluate neutrophil migration. Decreases in CXCL1-8 cytokine within the supernatant ought to lower the migration of the neutrophils from the higher to the decrease chamber.
CRISPR engineering Ok-562 MYC/d2GFP cell line
Ok-562 cells have been modified to co-express d2GFP together with MYC as beforehand described18. Briefly, beforehand printed MYC homology arms surrounding the P2A-d2GFP cassette have been bought from Azenta and cloned into the pUC-GW-Amp vector. An sgRNA was ordered from IDT to focus on the MYC 3’ UTR (mC*mU*mU*rGrUrGrCrGrUrArArGrGrArArArArGrUrArGrUrUrUrUrArGrArGrCrUrArGrArArArUrArGrCrArArGrUrUrArArArArUrArArGrGrCrUrArGrUrCrCrGrUrUrArUrCrArArCrUrUrGrArArArArArGrUrGrGrCrArCrCrGrArGrUrCrGrGrUrGrCmU*mU*mU*rU). RNPs have been shaped by combining equimolar ratios of sgRNA and recombinant spHiFi Cas9 Nuclease v3 (IDT, Cat# 1081060) protein and incubating at room temperature for 20 min. 1 million Ok-562 cells have been electroporated with 2 µg of HDR plasmid and 250 pmol RNP utilizing a Nucleofector 4D (Lonza) and the SF reagent package (Lonza, Cat# V4XC-2032) in accordance with the producer’s directions.
Cells have been allowed to recuperate and develop for 7 days, after which GFP + cells have been sorted 5 cells/effectively right into a 96-well plate by fluorescence-activated cell sorting (FACS, with a gating technique offered in Supplementary Fig 6). Clone swimming pools have been allowed to develop for a further 7 days after which have been genotyped by lysate PCR for the d2GFP insert utilizing primers directed to MYC exon 3 and the three’-UTR (Supplementary Information 1). Homozygously edited clonal swimming pools containing solely the modified allele, which weren’t heterozygous for the wild-type and modified allele, have been recognized by way of gel electrophoresis and chosen for a further PCR display screen to confirm correct integration utilizing primers directed to MYC intron 2 and d2GFP (Supplementary Information 1). Clone swimming pools have been subjected to a limiting dilution in a 96-well plate to establish single-cell clones that have been PCR genotyped with each assays to make sure a pure inhabitants.
Chromatin immunoprecipitation adopted by high-throughput sequencing (ChIP-Seq)
ChIP-seq was carried out by way of high-throughput ChIPmentation as beforehand described37. Briefly, 500,000 cells have been cross-linked in 1% formaldehyde for 15 min and quenched with 275 mM glycine previous to flash freezing and storage at −80C. p65 ChIP was carried out utilizing double-crosslinking with 1.5 mM EGS incubated for 30 minutes and spun down for five minutes at 5000 × g. The supernatant was discarded, and the pellet was resuspended in 1% formaldehyde. Samples have been then incubated for 10 minutes, spun down for five minutes at 5000 × g, and the pellet was flash frozen. Antibodies for HA (3 µg, EpiCypher, Cat# 13-2010), H3K9me3 (1 µg, Diagenode, Cat# C15410193), p65 (3 µl, Cell Signaling, Cat# 8242), or H3K27Ac (3 µg, Abcam, Cat# Ab4729) have been loaded onto Protein G Dynabeads (1009D, Life Applied sciences) in PBS and 0.5% BSA for 4 h, rotating at 4 oC. Mounted cells have been thawed in Cell Lysis Buffer (50 mM Tris HCl, 10 mM EDTA, 0.5% SDS) and HALT Protease Inhibitor (Thermo Fisher Scientific, Cat# 87786) after which processed by way of multi-modal shearing utilizing a probe-based sonicator (QSonica) adopted by the PIXUL acoustic Sonicator (Lively Motif). An enter fraction was reserved, and cell lysates have been transferred onto pre-loaded antibody-bead conjugates for immunoprecipitation in a single day, rotating at 4 oC.
Following immunoprecipitation, beads have been washed previous to on-bead tagmentation of chromatin utilizing Tagment DNA Enzyme 1 (Illumina, Cat# 20034197). Each immunoprecipitate and enter fractions have been tagmented for 10 min at 37 oC with shaking. Samples have been washed and PCR amplified for 12 cycles utilizing HiFi MasterMix (New England Biolabs, Cat# M0541) and combinatorial i5/i7 primers derived from Mezger A, et al.38. The PCR protocol included an preliminary 5 min 72 oC incubation for gap-filling and a 5-minute incubation at 95 oC, adequate for cross-linking reversal. Remaining libraries have been purified utilizing SPRI beads (1X SPRISelect, Beckman Coulter, Cat# B23318), pooled at equimolar ratios, and sequenced on a NextSeq 2000 Sequencing System (Illumina) utilizing a 2×50 PE technique. Every library was sequenced, concentrating on a depth of 30 M uncooked reads/fragments.
Illumina sequencing knowledge in FASTQ format was collected and QCed utilizing FASTQC39 to make sure the NGS high quality of reads earlier than beginning the ChIP-Seq evaluation pipeline. Illumina sequencing adapters have been trimmed utilizing Trimmomatic (v0.6.7) to remove technical sources of ChIP-Seq bias. Trimmed reads have been aligned to human genome hg19 utilizing Bowtie240 to create the preliminary mapped BAM recordsdata41. Mapped reads within the BAM recordsdata have been sorted and filtered to take away duplicate reads within the generated duplicated BAM recordsdata utilizing Picard42 (v3.1). MACS243 (v2.2.7.1) was utilized on filtered BAM recordsdata of particular person ChIP samples to name ChIP peaks utilizing FDR
3’ DGE RNA-Seq
3’ Digital Gene Expression (DGE) RNA-seq was carried out utilizing the Lexogen QuantSeq 3’ mRNA-seq V2 package (Lexogen Cat# 191.24) in accordance with the producer’s directions. Briefly, 250 ng of whole RNA was enter into first-strand synthesis utilizing oligo(dT) priming and subsequent RNA removing. Second-strand synthesis was carried out utilizing random priming adopted by cDNA purification by way of SPRI-beads. Library amplification was carried out utilizing 12 nt UDIs over 17 PCR cycles and purified by way of SPRI-beads. Libraries have been sequenced on a NextSeq 2000 utilizing a 1 x 100bp technique.
Information was processed in accordance with the producer’s tips (QuantSeq 3‘ mRNA-Seq Built-in Information Evaluation Pipelines on Bluebee® Genomics Platform 015UG108V0201) Briefly, UMItools (v1.1.4) was used to extract UMI barcodes and adapters have been trimmed utilizing TrimGalore (v0.6.10) and aligned to the mm10 reference genome utilizing STAR (v2.7.11b). The deduplicated bam recordsdata are used because the inputs to HTSeq (v2.0.5) for quantification. Samples with lower than 10 million reads passing QCs have been filtered. Uncooked gene-level rely knowledge have been imported utilizing tximport, and differential expression was calculated utilizing DESeq2 (v1.40.2) with the ~ timepoint + remedy for the experiment-wide evaluation.
Complete Genome Methylation Sequencing
Complete genome methylation sequencing libraries have been ready utilizing the NEB Enzymatic Methyl-Seq Equipment (NEB Cat #E7120L) in accordance with the producer’s directions. Briefly, gDNA was acoustically sheared utilizing the PIXUL (Lively Motif) to realize ~ 300 bp fragments utilizing the instructed protocol: Pulse 50 N/PRF 1 kHz/Burst 20 Hz/Time 36 min. 200 ng of sheared gDNA was finish repaired and ligated with EM-seq adapters. Following SPRI bead purification, adapter-ligated DNA was enzymatically transformed. Purified, transformed DNA was dual-indexed and amplified by PCR utilizing Q5U polymerase for 4 cycles. Remaining libraries have been size-selected utilizing a 0.85X SPRISelect bead purification, pooled at equimolar ratios, and sequenced on a NextSeq2000 utilizing a 2x150bp technique. Bismark (v0.24.0) was used to align uncooked sequencing reads to a transformed hg19 genome and estimate the CpG methylation stage. DMRSeq (v1.26) was used to establish differentially methylated areas (DMR) between the remedy teams from the uncooked methylation estimate. The ‘adjustCovariate’ parameter was set to regulate time factors.
Amplicon methylation sequencing
Genomic DNA was normalized to 200 ng in 100 µl low Tris-EDTA (TE) buffer and sheared briefly utilizing the PIXUL (Lively Motif) to acquire fragments lower than 15 kb in dimension: 5 Pulse/1 kHz PRF/3 min/20 Hz Burst. Fragmented DNA was purified utilizing 1X SPRI-Choose (Beckman-Coulter) and subjected to EM-conversion utilizing the EM-seq Conversion Equipment (New England Biolabs, Cat# E7125) in accordance with the producer’s directions. Purified, transformed DNA was PCR amplified for 40 cycles on the locus of curiosity utilizing Q5U MasterMix (New England Biolabs, Cat# M0597) in accordance with the producer’s directions utilizing the primers in Supplementary Information 1 (500 nM every in 20 µl reactions).
Following SPRI bead purification (1.8X SPRISelect, Beckman Coulter), the amplicon was transposase-labeled with Illumina sequencing adapters utilizing Tagment DNA Enzyme 1 (20034197, Illumina). Tagmentation was carried out utilizing 0.1 µl enzyme per 10 µl response containing roughly 30 ng of the amplicon for five min at 37 oC, and the response was stopped with 0.04% SDS. Libraries have been dual-indexed (combinatorial) by way of PCR utilizing KAPA HiFi ReadyStart MasterMix (KK2602, Roche Sequencing Retailer) and i5/i7 primers derived from Mezger A, et al.38. PCR reactions occurred in 40 µl volumes with 100 nM of every primer for 13 cycles.
Remaining libraries have been purified utilizing SPRI beads (1X SPRISelect, Beckman Coulter), pooled at equimolar ratios, and sequenced on a MiSeq System (Illumina) utilizing a v2 Nano 2x150bp reagent package (MS-103-1001, Illumina). The sequencing reads have been trimmed, and Bismark44 was used for learn alignment and quantification. The imply methylation values have been estimated primarily based on the methylated ratio of particular person CpGs.
Circulate cytometry and sorting of Ok-562 MYC/d2GFP cells
Ok-562 (wild-type) and Ok-562 MYC/d2GFP cells have been grown in full media (RPMI-1640; 11875, Gibco + 10% FBS; S11550, Atlanta Biologicals + 1% Pen/Strep; 15140, Gibco) and three million cells have been seeded into T75 flasks. Cells have been handled with MYC-EC or JQ1 (4499; Torcis) on the specified concentrations by direct addition to the media; separate flasks have been used to tradition single-color controls (untreated Ok-562 MYC/dsGFP and MYC-EC-treated Ok-562 wild-type). MYC-EC-treated and JQ1-treated cells have been harvested after 44 h and 16 h, respectively, by centrifugation at 400 × g for five min and resuspended in PBS.
In dosage, titration experiments, cell suspensions have been straight plated (200 µl/effectively) in a spherical backside 96-well plate (3879; Costar) and analyzed on an Aurora circulate cytometer (Cytek). 100,000 occasions have been collected from three unbiased experiments. Cells have been gated to establish dwell, single-cells primarily based on FSC/SCC, and GFP populations have been decided by evaluating histograms of untreated controls with the MYC-EC 4 µg/ml situation.
In sorting experiments, cell suspensions have been filtered by means of a cell strainer (352235; Falcon) previous to sorting on an MA900 cell sorter (Sony Biotechnology). A 130 µM chip (LE-C3213; Sony Biotechnology) and automatic setup beads (LE-B3001; Sony Biotechnology) have been used to ascertain optimum instrument settings for gating and sorting. Cells have been gated to establish dwell, single-cells primarily based on FSC/BSC, and GFP-/tdTomato + or GFP + /tdTomato- cells have been sorted and picked up off a biaxial plot (eGFP vs. tdTomato). An unsorted inhabitants was reserved previous to loading onto the sorter. The genomic DNA of collected cells was extracted utilizing the DNeasy Blood & Tissue Equipment (69516; Qiagen), and all the eluate was used as enter for amplicon methylation sequencing. FlowJo 10.8.1 (BD Biosciences) was used for cytometric evaluation.
Laboratory animals used on this manuscript
C57BL/6 regular (Jackson Labs inbred B6; pressure #000664) mice (Mus musculus) have been used within the Pcsk9 and Cxcl research on the ages described in every experiment. Transgenic Ai14 (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, Jackson Labs pressure #007914) mice (Mus musculus), aged 6–8 weeks previous, have been used within the LNP biodistribution research. Animals have been housed in teams of 5 animals per cage, with AlphaDri®, wooden shavings, or corn cob as bedding, which was modified as soon as per week at minimal. Animals have been acclimatized previous to the graduation of every research, throughout which the animals have been noticed to establish any that have been current in poor situation. The research have been carried out at services offered by Pharma Fashions, MLM Medical Labs, or Biomere Biomedical Analysis Fashions, Inc., the place animal rooms have been saved at a temperature of 70 ± 5 °F and 50% ± 20% relative humidity with 10–15 air modifications per hour. The room offered cycles of 12 h gentle adopted by 12 h darkish with no twilight. Every facility retained information of temperature and relative humidity. Animals have been fed with Envigo 2920X or equal business sterile rodent chow. Sterile water was offered advert libitum.
In vivo Pcsk9 pilot research
Forty feminine C57BL/6 mice (roughly 14 weeks previous) have been subdivided into 4 teams (10 mice every) and have been handled by way of tail vein injection with the indicated dose of the EC or PBS management. At 7 days post-administration, serum and liver punches have been remoted. Liver punches have been homogenized, and RNA was remoted. RNA was then transformed to cDNA. cDNA was analyzed by multiplexed qPCR utilizing TaqMan probes particular to Hprt1 (housekeeper) and Pcsk9. Relative Pcsk9 mRNA expression was decided by means of the comparative delta-delta Ct technique. Serum Pcsk9 ranges have been detected utilizing the Mouse Pcsk9 ELISA Equipment (ab215538, Abcam). This research was carried out at Pharma Fashions (Marlborough, MA).
In vivo Pcsk9 long-term research
140 feminine C57BL/6 mice (roughly 6 to 7 weeks previous) have been subdivided into 2 teams (70 mice every) and have been handled by way of tail vein injection with 3 mg/kg of the EC or PBS management. Serum samples have been collected each two weeks and analyzed for Pcsk9 content material. Ten mice from every group have been sacrificed beginning at 14 days post-injection, then month-to-month by means of six months. Liver punches have been remoted and analyzed for Pcsk9 promoter methylation by amplicon methylation sequencing. This research was carried out at Pharma Fashions (Marlborough, MA).
Cxcl1-8 In vivo read-outs
Feminine C57BL/6 mice (roughly 14-weeks previous) acquired a 1 mg/kg iv administration of the MM-Cxcl-EC1/MM-Cxcl-EC2 mixture or GFP management in an LNP formulation 8 h previous to the LPS problem, 10 mice per arm. 50 uL of LPS was administered by oropharyngeal aspiration into every animal at 0 h. Mice have been monitored and samples of BALF and peripheral blood have been collected at 24 h after LPS problem for circulate cytometric evaluation. Utilizing the antibodies Ly6G-FITC (dil, Biolegend, Cat# 127605), CD45-BV605 (dil, Biolegend, Cat# 103155), B220-PE-Cy7 (dil, Tonbo, Cat# 60-0452), SiglecF-BV421 (dil, Biolegend, Cat# 155509), CD3-PE (dil, Biolegend, Cat# 100205), CD11b-BV785 (dil, Biolegend, Cat# 101243), and CD11c-Percp/Cy5.5 (dil, Biolegend, Cat# 117327) and the gating technique illustrated in Supplementary Fig 7, neutrophils have been recognized as CD45 + , Siglec F-, CD11b +, CD11c-, Ly-6G +, T cells have been recognized as CD45 + , Siglec F-, CD11c-, CD3 +, and B cells have been recognized as CD45 +, Siglec F-, CD11c-, B220 +. Physique weights have been measured on the day of remedy and 1-day pre and post-treatment for ten animals per group. For Immunohistochemistry (IHC), one animal from every group was used to look at morphologic pathology of the lung. Lungs have been inflated and glued with formalin and H&E staining for irritation and cell infiltration evaluation. This research was executed at MLM Medical Labs, Inc. (Minneapolis, MN).
LNP Biodistribution research
Three feminine Ai14 mice, 6–8 weeks-old, have been dosed intravenously with 3 mg/kg of Cre mRNA (TriLink, Cat# L-7211) loaded into DOTAP LNPs for general evaluation of LNP biodistribution, with a qualitative evaluation of sections primarily based on cell morphology. The liver, lungs, and spleen have been dissected 48 h post-LNP remedy for quantification of tdTomato reporter protein exercise. The organs have been imaged at 554/581 Ex/Em (nm) in a Perkin Elmer IVIS Spectrum S3 Imaging System. tdTomato exercise was expressed as radiance (photons/sec/cm2/steradian). This research was carried out at Biomere Biomedical Analysis Fashions, Inc. (Worcester, MA).
The dissected organs have been fastened for twenty-four h in 10% Impartial Buffered Formalin. Tissues have been additional dehydrated in Ethanol and embedded into paraffin blocks for immunohistochemistry (IHC) evaluation. Rabbit anti-RFP antibody (1:300 dilution, Novus Biologicals, Cat# NBP1-72732C) and anti-rabbit HRP/DAB micro-polymer antibody (Abcam, Cat# Ab236469) have been used as main and secondary antibodies, respectively. A peroxidase-based detection package (ImmPACT®, Cat# SK-4103) was used to develop the colour of tdTomato reporter protein within the tissue.
Statistics and reproducibility
For every in vivo research, the precise numbers and strains of mice used are listed within the technique and determine legends. Mice have been randomized into management and remedy teams previous to remedy. No statistical technique was used to predetermine the pattern dimension. The numbers of animals used have been adequate to generate adequate statistical evaluation (utilizing one-way or two-way ANOVA) on the finish of remedy intervals. For every in vivo research, the research director on the animal facility weren’t blinded to the remedy modality. Outcomes are introduced from one unbiased research every. For the in vivo research, ROUT outlier detection strategies have been utilized.
All in vitro experiments have been repeated in higher than or equal to 2 organic replicates with higher than or equal to 2 technical replicates included. Particular replicates are listed in determine legends. In vitro experiments weren’t randomized. For in vitro experiments, the investigators weren’t blinded to allocation throughout experiments and final result evaluation. Statistics for figures have been calculated utilizing GraphPad Prism (model 10.4.1) algorithms and have been one- or two-way ANOVA checks or t checks as indicated in particular person determine legends. All t checks have been unpaired, two-tailed except in any other case indicated within the determine legend. A number of-testing was carried out (BH-FDR) when a number of t checks have been examined and verified to be q
Statistical significance and impact sizes for differentially methylated areas (Desk 1) have been calculated utilizing the R (v4.3.1) package deal DMRSeq45 (v1.26) the place beta represents the coefficient worth for remedy impact. DMRs have been filtered primarily based on an adjusted p-value threshold of 0.05 calculated utilizing the Benjamini-Hochberg technique (BH-FDR).
Statistical significance and Log2-fold-change (log2FC) of normalized counts for differentially expressed genes have been calculated utilizing the R package deal DESeq246 (v1.40.2) utilizing the design “~ timepoint + remedy” for the experiment-wide evaluation and a BH-FDR adjusted p-value of
Reporting abstract
Additional info on analysis design is obtainable within the Nature Portfolio Reporting Abstract linked to this text.